Clinical Trials
In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.
Spain has one of the highest incarceration rates in Europe, with 160 individuals imprisoned per 100,000 residents. In 2020, 43,494 people were incarcerated in facilities not managed by regional health authorities — essentially all regions except Catalonia, the Basque Country, and Navarra.
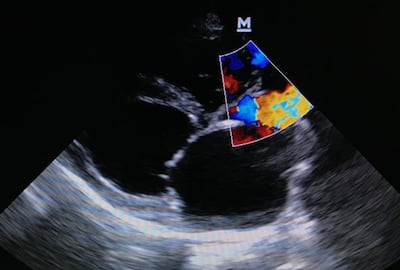
TRiCares announced first implantation of the Topaz transfemoral tricuspid heart valve replacement system as part of the company’s EU pivotal study. If all goes to plan, the device will compete with Edwards’ Evoque system. The announcement follows the company’s $50m series D funding raise in July.
Tim Schmid, executive VP and worldwide chairman of J&J MedTech, expects Shockwave, acquired in April, and Abiomed, purchased in late 2022, to be “long-term gems” for the company. Cardiovascular is among higher-growth segments where J&J has concentrated investments in recent years, along with robotic surgical systems.
The US FDA has published a trio of draft guidance documents for its Assessment Scheme for Conformity Program, which began as a pilot in September 2020 to capitalize on the role of standards in the regulation of medical devices.
Click Therapeutics reports positive Phase 3 clinical data for CT-132, a digital therapeutic intended as an adjunctive treatment of episodic migraine. The company is pursuing FDA clearance and actively exploring opportunities to offer a drug-digital combination therapy with added clinical benefit that provides “a single, integrated experience for the patient, prescriber and payer.”
This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.
A key differentiating feature between Medtronic’s Aurora extravascular ICD and Boston Scientific’s Emblem subcutaneous ICD, Aurora’s anti-tachycardia pacing was successful 77% of the time, in line with transvenous ATP rates, and shocks were avoided in nearly half of spontaneous arrhythmic episodes due to the availability of ATP, across average pivotal trial follow-up of 30.6 months.
Inari Medical is updating use instructions for a clot-removing catheter due to the potential for serious adverse effects, including death.
The Acton, MA-based tubeless insulin pump specialist expands its indication for the Omnipod 5 AID system beyond type 1 diabetes as FDA authorization for type 2 comes sooner than Wall Street expected. Analysts expect clearances of rival systems from Tandem Diabetes Care and Medtronic in 2025, but believe Insulet is well-positioned to compete.
Two years after the EU adopted the original common specifications for certain products under the IVD Regulation, the commission has added new products and updated its requirements.
Medtech Insight interviewed Meri Beckwith, co-founder of “anti-CRO” clinical research organization Lindus Health, about his company’s efforts to make clinical trials more efficient and reliable.
J&J buys heart failure implant company V-Wave, whose Ventura Interatrial Shunt could be the first device of its kind aimed to reach the roughly 800,000 patients in the US who experience heart failure and reduce ejection fraction every year.
Caresyntax said it will use the $180m it recently raised in a series C extension and debt financing round to build out its vendor-neutral surgery platform aimed to help surgeons with real-time and long-term decision support to improve patient outcomes and efficiencies.
Hello Heart has introduced a symptom tracking feature in its app, allowing users to log feelings of dizziness or shortness of breath in conjunction with blood pressure readings. The enhancement will help all users to monitor cardiovascular risks, but women in particular could benefit, the company suggests.
The Seattle-WA-based company’s latest capital raise follows study results published in July in which Know Labs’ proprietary non-invasive RF dielectric sensor and machine learning algorithms correctly classified participants’ glycemic status as hyperglycemic, normoglycemic, or hypoglycemic with 93.37% accuracy compared with venous blood glucose values. Know Labs' goal is to commercialize a diabetes screening device that could help to funnel undiagnosed patients into the health care system.
Global investment firm Carlyle has agreed to pay $3.8b for Baxter International’s Kidney Care unit. The newly spun-off business will be known as Vantive.
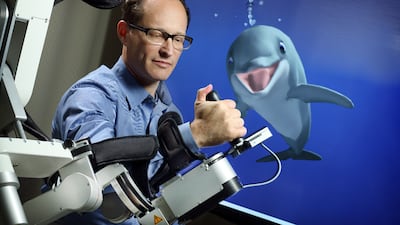
MindMaze seeks to create a beautiful immersive experience for patients recovering from brain injuries. Chief medical director John Krakauer, clearly a “Star Wars” fan, discusses the significant need for digital therapeutics at a time when therapists are in short supply, as well as the challenges facing the DTx space as a whole.
The US FDA has published a discussion paper as part of an effort to ensure all patients have timely access to safe and effective medical devices. The agency says the paper fits into a larger strategy of promoting health equity and is seeking public comment on the initiative.
Biolinq’s CEO Rich Yang spoke to Medtech Insight about the company’s wearable patch in development, which uses tiny microsensors to measure, for now, glucose, with ample runway for additional indications down the line. If approved by the US FDA, the device would become the first of its kind to monitor glucose levels in diabetes patients not using insulin.