Armenia
Country
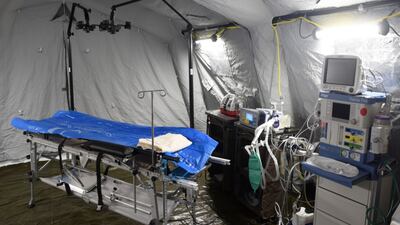
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have since stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
Russia and Ukraine had been key players in the pharma markets of Central Asian and surrounding countries before the launch of the Russian invasion into its neighbor on 24 February. Because of this, both countries are poised to give ground in this growing regional market.
After a five-year regulatory transition, access to the Eurasian Economic Union market for new medtech products must now take place via the EAEU system only. But having got off to a slow start, further system transitional measures are now under discussion.
The Eurasian Union’s medical device regulatory system, many years in the planning, becomes mandatory in January 2022. But new amendments posted in late summer allow for national registrations to remain valid after the deadline. Moscow-based consultancy RegMT explains the background, and the likely path ahead.
There are just 4.5 months left until the new harmonized Eurasian Economic Union medtech system becomes mandatory, signifying major changes in regional market access. EAEU member Russia is meanwhile strengthening its post-market medtech controls.
Stada will pay $660m for a basket of 20 brands from Takeda to expand its consumer health portfolio in Russia and a number of strategically important CIS markets.
The new regulatory framework in the Eurasian Economic Union is now in place and the first drug approval applications have been filed under the mutual recognition system. Applications are expected to rise significantly in 2019 once IT systems are fully in place and more experience has been gained.
It might seem that the Eurasian Economic Union (EAEU) agreement on medical device regulation is nothing but administrative issues and slow progress, but it should deliver benefits to all stakeholders after launch, currently slated for 2022. but manufacturers need to be mindful of the member states' strengths and weaknesses when selecting their reference member state.
The transition deadline for adoption of Eurasian Economic Union (EAEU) medtech principles across the five member states remains the end of 2021, but vital elements are still not resolved, giving rise, for the first time, to notions of a delay in adoption. This would be the pragmatic course, local market experts believe, but for now, efforts are aimed at completing the system on time.
The much-anticipated Eurasian Economic Union (EAEU) was originally targeted to come into effect at the start of 2016, but more than year later, the five-member trading bloc has yet to be ratified by all nations. The Russian medtech industry is impatient for progress, but IMEDA's Sergey Kolosov sees some promising signs in recent developments.
The Armenian government is drafting a bill that should cap drug prices, the country's health minister, Arutiun Kushkyan, has said. The move follows significant price increases by the industry at the time of swine flu epidemic last autumn.
Armenia is set to follow other ex-Soviet republics in its intention to impose GMP standards at local pharmaceutical enterprises. Ara Babloyan, chairman of the parliamentary health committee, said that the country's parliament is planning to consider and adopt a corresponding legislative package.
ADVERTISEMENT