Blood Disorders
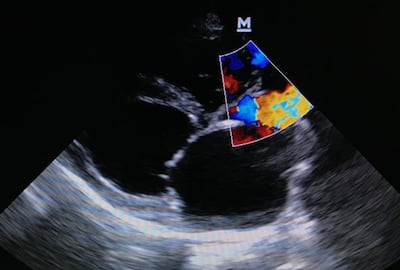
TRiCares announced first implantation of the Topaz transfemoral tricuspid heart valve replacement system as part of the company’s EU pivotal study. If all goes to plan, the device will compete with Edwards’ Evoque system. The announcement follows the company’s $50m series D funding raise in July.
The second instalment of In Vivo’s three-part series delves into the therapeutic categories that will propel forecast pharmaceutical sales growth for 2025, focusing on blood malignancies, skin conditions and generalized cardiovascular disease.
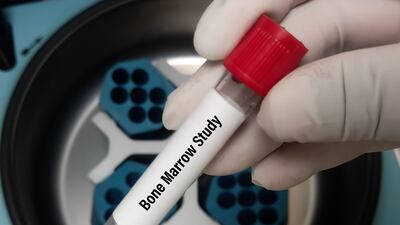
The US FDA has cleared a novel software application to enhance the analysis of bone marrow smears. Using the AI-powered tool, hematopathologists may be able to better diagnose various blood and marrow diseases.
The US FDA is advising the public that certain plastic syringes from China could fail, leak, or break. If necessary, the agency may prevent Chinese syringes from entering the US.
As the US FDA works to finalize new regulation of lab-developed tests, it must consider more than two thousand comments that have poured into the agency since the proposed rule was published in October. The comment period closes Monday.
This week, the US FDA labelled several recalls class I, approved an at-home diagnostic for chlamydia and gonorrhea, and announced the expansion of its TAP pilot program. Guardant Health also announced it would appeal a verdict in a patent suit against the company and AdvaMed voiced its continued support for pending breakthrough device legislation.
This week, the US FDA announced that several pandemic-era guidance documents are no longer in effect; an FDA advisory committee meeting on multi-cancer detection (MCD) diagnostics was scheduled; and both a needle-free blood collection device and the first VR treatment for pain and anxiety landed FDA clearances.
The FDA radiological devices advisory panel said that there wasn’t enough evidence backing the use of irradiation to prevent metastasis in the blood of cancer patients, especially given the treatment’s known risks.
This week, FDA cleared Terumo's Reveos automated whole blood processing system, Abbott adds the Alinity-h hematology system to its comprehensive lab diagnostics lineup, Movano moves forward with "smart ring" technology, and more.
Innovation is a key to long-term success for biopharma companies, but building the right climate to foster creative problem-solving is challenging. Questioning assumptions and constraining the target of innovation can propel a company forward.
Dewei Medical Equipment Co. is recalling thousands of DNA/RNA preservations kits because they were distributed in the US without FDA clearance.
A CDC study found that fewer than half of children with sickle cell disease were getting screened for strokes, and fewer than 40% of children were taking recommended medications.
Two Massachusetts-based infusion pump manufacturers with the same founder and CEO received warning letters from the US FDA for similar violations.
The company has developed a device for extracorporeal blood filtration that is compatible with dialysis machines.
The Dutch firm expects the financing to advance its novel asset Microlyse into a potentially pivotal study for a rare blood disorder.
The New York State Department of Health Clinical Laboratory Evaluation Program (CLEP) has cleared an innovative test from life sciences company Karius that can detect more than 1,000 pathogens from all five classes.
Citing the pandemic, the US Centers for Medicare & Medicaid Services (CMS) discounted 2020 data in reviewing renewals for New Technology Add-On Payment (NTAP) status and granted extensions through 30 September to 13 NTAP products.
The company stopped shipping Alaris in early 2020 after the FDA requested a new 510(k) for modifications and the device has been the subject of several recalls since then.
Elizabeth Fowler of the Commonwealth Fund will likely lead the CMS Innovation Center, which tests health care payment systems.
A telemedicine legislative expert explains provisions of bills that would expand reimbursement provisions in Congress.
ADVERTISEMENT