Bulgaria
Country
Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.
J&J says it has “exhausted all current viable avenues” to get its antidepressant nasal spray Spravato reimbursed on England’s National Health Service, after NICE decided against re-appraising the drug following numerous funding rejections.
Belgium's Ceres Pharma has expanded its footprint in Eastern Europe by acquiring supplements and OTC device specialist ABOPharma.
The practice of telehealth got the impetus it had long needed during the pandemic, as experiences in Czechia and neighboring economies in eastern Europe show in a regional roundup by Kinstellar.
Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.
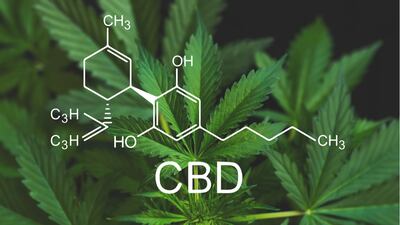
INFOGRAPHIC: An interactive timeline of HBW Insight's coverage of the blossoming European CBD market in 2019. The timeline covers the main events of the year beginning with the European Commission's decision to classify CBD as a novel food, and ending on the filing of the first application for a synthetic form of the cannabis-derived ingredient.
Bulgaria should remove clawback taxes for generics and biosimilars, avoid external reference-pricing mechanisms, adopt measures to drive up generic prescribing and adopt a “dynamic” biosimilars policy to encourage uptake, European off-patent industry association Medicines for Europe has urged. The industry body has also called on the new European Commission to offer the country its support in these areas.
Stada is set to snap up Czech Republic-based Walmark in its second major deal this year as it looks to make good on its promise to rapidly expand its OTC-focused Branded Products business.
Medical Marijuana Inc. says claims that it has lost its licence to sell CBD as a food supplement in Bulgaria are incorrect. The firm tells HBW Insight it has in fact been granted additional approval to export CBD products from Bulgaria to Russia.
Bulgaria has granted US-based Medical Marijuana Inc. the right to sell CBD as a supplement in a first for an EU country. The move, which will see CBD regulated as a traditional food, puts Bulgaria directly at odds with the European Commission, which recently classified the cannabis plant extract as a novel food.
The number of European countries whose drug manufacturing site inspections the US regulator can rely on in place of its own inspections has grown.
Stay up to date on regulatory guidelines from around the world, with the Pink Sheet's Guidance Tracker
Five countries appear to be ahead in the battle to host the European Medicines Agency after Brexit, judging by the results of a European Commission assessment and an EMA staff survey, although much horse-trading among member states is expected before the winner is announced in November.
The European Medicines Agency says it could lose more than 70% of its staff if it moves to one of the cities least favored by current employees. In the worst-case scenario, the EMA says it would be unable to operate and that the regulatory system would “unravel.”
Teva's opportunity to compete in the US OTC drug market now that the Allergan's generic nonprescription products are under its roof makes company "more excited" about the space "than we have ever been," says CEO Sigurdur Olaf Olaffson,
Teva's opportunity to compete in the US OTC drug market now that the Allergan's generic nonprescription products are under its roof makes company "more excited" about the space "than we have ever been," says CEO Sigurdur Olaf Olaffson,
Guido Rasi's decision to step aside as executive director of the European Medicines Agency, the result of a procedural dispute between the EU authorities and the former head of the Bulgarian medicines agency Emil Hristov, is a big blow to the agency and comes at a particularly bad time for the European Commission after the recent resignation of the head of its health directorate.
MannKind has said that it reached a final resolution of the arbitration proceedings initiated by John Arditi, the company’s former senior director of good clinical practices within the regulatory affairs group who had sued the company claiming he was retaliated against for raising concerns about the clinical trials for the inhaled insulin Afrezzo (insulin human rDNA origin) conducted in Russia and Bulgaria.
Dr Kiril Dobrev has been appointed deputy health minister of Bulgaria after Dr Mihail Zortev's resignation. Dr Dobrev's most recent position was director of the university hospital in Stara Zagora, central Bulgaria.
MannKind, the US company focusing on R&D and commercialisation of diabetes and cancer treatments, has denied that clinical trials of Afrezza, its diabetes drug candidate, in Russia and Bulgaria did not comply with standards. The misconduct allegations were made by John Arditi, a former regulatory affairs executive, who is suing MannKind for wrongful termination of his employment.
ADVERTISEMENT