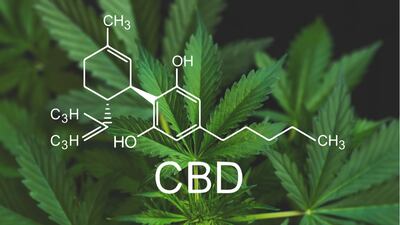
Cannabidiol CBD
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.
Noting similar warnings a year ago, the FDA and FTC announce warnings to six more, part of a joint effort to stop sales of copycat food products containing delta-8 THC, saying companies selling these "illegal products are demonstrating complete neglect for consumer safety.”
Committee’s report published with FY2025 appropriation states a different approach to establishing FDA regulation of non-drug products containing hemp as a derivative of cannabis de-scheduled as controlled substance in the US since 2018.
Industry expects instructions stated in committee’s report to counter an amendment included in the bill it passed by on 10 July which would define as controlled substances a majority of hemp ingredients currently available in supplements, food and non-drug topicals in the US.
CV Sciences closes purchase of Elevated Softgels, a manufacturer of encapsulated softgels and tinctures for the supplement and nutrition industry. Captek Softgel starts gummy supplement production at a 60,000-square foot facility.
With a first-in-class cannabis-derived treatment for epilepsy, a market-leading treatment for sleep disorders and now a foray into movement disorders, Jazz’s CNS pipeline is strong.
The European Federation of Associations of Health Product Manufacturers, EHPM, is hopeful the upcoming EU elections will trigger action on key issues related to dietary supplements, including novel food authorizations and harmonized vitamin levels.
Where a US hemp product firm stands on whether de-scheduling the botanical as a controlled substance in the 2018 farm bill left a loophole allowing chemically derived and potentially intoxicating cannabinoid strains to qualify could determine whether it agrees with the detour.
Ag committee members in 23 May markup likely to broach topic of delta-9 THC limit for hemp. Since hemp was de-scheduled in 2018, cannabinoids other than delta-9 but with psychoactive effects have become leading sales drivers. “Most states now have legal marijuana programs, they just don't know it,” says cannabis industry attorney.
DEA NPRM to move marijuana from CSA Schedule I to Schedule III says synthetically derived THC “is outside the CSA’s definition of marijuana” and “will remain in Schedule I.” Synthetically derived THC includes copies of ingredients derived from cannabis plants which qualify as hemp by containing no more than 0.3% delta-9 THC concentration by dry weight and are common in supplements and food available in US.
CBD products produced by Chanelle McCoy Health and Cannaray have been given the green light by the Food Standards Agency's Advisory Committee on Novel Foods and Processes allowing them to progress to the final stages of the regulator's lengthy approval process. Meanwhile, the Home Office has changed its policy with regards to THC in CBD, in response to an action brought against it by Jersey Hemp, clearing another barrier to making the UK market compliant.
Litigation the court agreed to accept for its term beginning in October doesn’t allege CBD in a tincture purchased in 2012 contained an unlawful level of delta-9 THC but contained any THC at all. Also, FDA Commissioner Califf encourages Congress to authorize regulatory pathway for lawful use of CBD and other hemp ingredients in supplements and food.
House appropriations subcommittee chair Andy Harris, R-MD, says the agency ignored several important factors in recommending that marijuana be reclassified as a Schedule III drug; Califf tells ranking member Sanford Bishop, D-GA, that although work is progressing on animal testing alternatives, 'we're a long way right now' from eliminating animal studies before first-in-human trials.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Without FDA regulatory pathway for hemp’s lawful use in supplements and food, some states are imposing more-stringent regulations while others allow sales of intoxicating ingredients not considered controlled substances as hemp derivatives.
US Cannabis Council says farm bill reauthorization is “key opportunity to tackle the national crisis caused by unregulated intoxicating hemp products” by limiting the variety of hemp derivatives which qualify as de-scheduled.
UK Food Standards Agency proposes shaving a minimum of three months off novel food authorization procedure as part of wider plans to shake-up the process for products like CBD supplements.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
ADVERTISEMENT