Cardiology
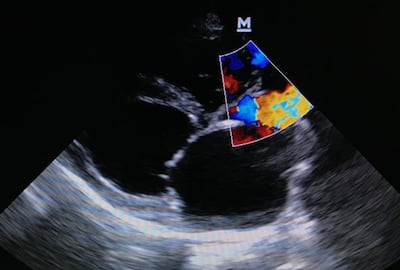
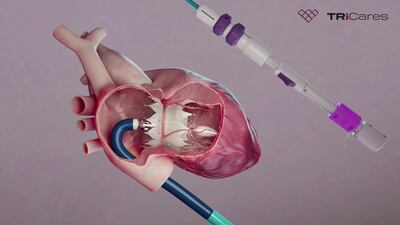
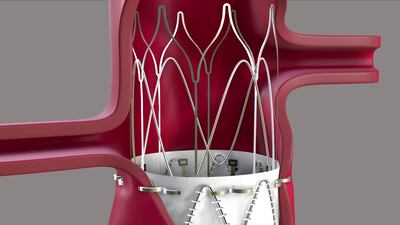
TRiCares announced first implantation of the Topaz transfemoral tricuspid heart valve replacement system as part of the company’s EU pivotal study. If all goes to plan, the device will compete with Edwards’ Evoque system. The announcement follows the company’s $50m series D funding raise in July.
Tim Schmid, executive VP and worldwide chairman of J&J MedTech, expects Shockwave, acquired in April, and Abiomed, purchased in late 2022, to be “long-term gems” for the company. Cardiovascular is among higher-growth segments where J&J has concentrated investments in recent years, along with robotic surgical systems.
This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.
The firm’s dual-chamber leadless pacemaker system with is expected to be a growth driver for Abbott and already has acquired approximately 30%-40% of market share from Medtronic since its approval by the US FDA in July 2023, according to GlobalData.
A key differentiating feature between Medtronic’s Aurora extravascular ICD and Boston Scientific’s Emblem subcutaneous ICD, Aurora’s anti-tachycardia pacing was successful 77% of the time, in line with transvenous ATP rates, and shocks were avoided in nearly half of spontaneous arrhythmic episodes due to the availability of ATP, across average pivotal trial follow-up of 30.6 months.
The second instalment of In Vivo’s three-part series delves into the therapeutic categories that will propel forecast pharmaceutical sales growth for 2025, focusing on blood malignancies, skin conditions and generalized cardiovascular disease.
This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.
J&J buys heart failure implant company V-Wave, whose Ventura Interatrial Shunt could be the first device of its kind aimed to reach the roughly 800,000 patients in the US who experience heart failure and reduce ejection fraction every year.
Hello Heart has introduced a symptom tracking feature in its app, allowing users to log feelings of dizziness or shortness of breath in conjunction with blood pressure readings. The enhancement will help all users to monitor cardiovascular risks, but women in particular could benefit, the company suggests.
This week, Medtronic recalled a nerve monitoring system due to reports of false responses. The US FDA approved the first auto-injector for opioid-overdose, made by Purdue Pharma. The agency granted de novo authorization for Labcorp’s PGDx elio plasma focus Dx used by labs for genetic profiling. As of 7 August, 950 AI/ML devices have been approved by the FDA. EKO Health teamed up with LSU to help detect arrhythmias and murmurs in student-athletes.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
This week, Edwards announced that it has purchased JenaValve and Endotronix; a New Jersey lab has agreed to pay the government $5m for violating anti-kickback law; eCential Robotics’ spine platform made its debut for human use; and more.
TRiCares SAS announced it raised $50m in series D funding from a single unnamed investor. The funding will support the company’s upcoming US early feasibility study and EU CE mark clinical investigation for its transfemoral tricuspid heart valve replacement system, Topaz.
Magenta Medical is preparing to launch a pivotal trial for its Elevate percutaneous Left Ventricular Assist Device, armed with $105m in new funding announced on 23 July. The company’s minimally invasive, FDA-designated Breakthrough Device supports heart function during high-risk percutaneous coronary intervention procedures.
This week, Nipro Medical Corp. announced it will invest $397.8m to build a US-based production plant, generating 232 new jobs; both Baxter and Hamilton announced ventilator recalls; Imperative Care wins FDA clearance for its stroke catheter; Intelligent Ultrasound Group plc entered into a conditional sale and purchase deal to sell its Clinical AI business to GE HealthCare for £40.5m; RMI distributed 350m rapid test kits in the fight against HIV/AIDS; Jiangsu Shenli Medical Production Co., Ltd received a second FDA warning letter about quality and safety of plastic syringes.
Life Seal Vascular Inc. secures US National Science Foundation funding to develop its innovative aneurysmal sac sealing technology for endovascular aortic repair. The solution aims to reduce complications and reinterventions in EVAR procedures.
This week, Roche filed suit against Foresight Diagnostics and Stanford University over patent infringement; the former head of a COVID-19 test company was convicted of securities fraud; and Baxter announced a recall of Life2000 ventilators.
Endoron Medical won $10m in series A funding from European venture capital firm Sofinnova Partners and the European Innovation Council Fund. The capital will propel Aortoseal – Endoron's endograft stapling solution for abdominal aortic aneurysm – through clinical validation in its US Food and Drug Administration early feasibility study.
The program, which brings together innovators and device industry stakeholders, helps ease the path to market for novel devices.
AliveCor’s Kardia 12L ECG System with its KAI 12 AI technology is the first AI-powered technology that can detect heart attacks using a portable system with a reduced leadset.
ADVERTISEMENT