Cellular & Genetic
Stakeholders looking to gain deeper insights into potential cell and gene therapy candidates and optimize R&D strategies can employ several strategies to inform portfolio management decisions.
President Joe Biden signed an executive order the White House said represents the most comprehensive executive action to date on improving women’s health. The move follows the president’s call during his State of the Union for Congress to invest $12bn in women’s health research.
This week, the US FDA labelled several recalls class I, approved an at-home diagnostic for chlamydia and gonorrhea, and announced the expansion of its TAP pilot program. Guardant Health also announced it would appeal a verdict in a patent suit against the company and AdvaMed voiced its continued support for pending breakthrough device legislation.
Legislation introduced in the US Senate by Wisconsin’s Tammy Baldwin would beef up the nation’s flu preparedness by investing in new technology for vaccine and diagnostic development as well as treatments.
Having led the way to regulatory approval for Kymriah and now Ebvallo, Atara CEO Pascal Touchon reflects on the evolution of cell therapy and what his company is doing differently.
Welcome to In Vivo’s new AI-focused blog, BioBytes, providing news and insight on the growing role of artificial intelligence in biopharma. In this post, Illumina’s new large language model-based gene analysis tool could help drive uptake of its products.
The White House Office of Science and Technology Policy has released an updated national strategic framework outlining priorities and goals for federal investment in AI. Industry executive Peter Shen weighs in on the plan.
Ring Therapeutics is turning to a previously unexplored family of viruses to improve the safety and efficacy of gene therapy. The preclinical science suggests using these anelloviruses as vectors can deliver payloads precisely and without causing a significant immune response. Human trials will need to demonstrate the technology can live up to its disruptive potential.
The European Union’s antitrust commission blocked the re-acquisition of Grail by Illumina days after an FTC administrative law judge ruled in favor of the merger in the US.
The FDA will discuss the de novo request for opioid use disorder genetic test, AvertD.
Bisphosphonate drugs, commonly prescribed to treat osteoporosis, have been linked to a reduction in breast cancer metastases in women. Inbiomotion is developing a test to expand the population eligible for this adjuvant therapy.
The standard helps developers determine whether their products might exude harmful amounts of nitric oxide.
The settlement resolves a total of eight lawsuits between the companies on genetic sequencing technology and alleged antitrust violations.
The US FDA has given thumbs-up to a genetic test from PreventionGenetics, the first agency-approved class II molecular companion diagnostic that looks for genetic deficiencies associated with obesity.
The US Food and Drug Administration is warning patients and health care providers about the risk of false results from prenatal tests that screen for genetic abnormalities in fetuses.
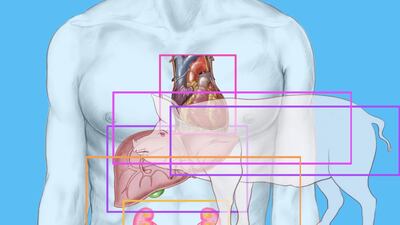
US surgeons have given new tailwind to xenotransplantation after successfully implanting a pig’s heart in a human. The breakthrough not only gives hope to the hundreds of patients who die annually for lack of a doner organ, but also to health care R&D departments who see the therapeutic value of transplanted tissues in tackling neurodegenerative disorders and diabetes.
Illumina insists its proposed $8bn acquisition of Grail, the developer of the Galleri multi-cancer test, would be “pro-competitive.” But antitrust regulators in the US government and European Commission suspect the deal would reduce competition and innovation in the future genomic diagnostics market.
The agreement will accelerate the development of cell-based therapies, where Thermo Fisher will build and run a development center
Illumina’s moves into infectious disease testing and further into the clinical setting have been accelerated by the pandemic. The genomic sequencing company is also fast expanding its companion diagnostics oncology partnerships.
At two recent confirmation hearings, HHS secretary nominee Xavier Becerra promised Senators he would help expand telehealth and procedure price transparency.
ADVERTISEMENT