Dietary Supplements
In this episode of the Over the Counter podcast, HBW Insight speaks to Kerry's RDA senior manager of immune and joint health, Sonja Nodland, about the opportunity for innovation that postbiotics represent for the consumer health industry.
Proposal to have panelists rate the strength of efficacy and safety evidence on a numeric scale also draws support in written comments on ways to optimize the advisory committee process.
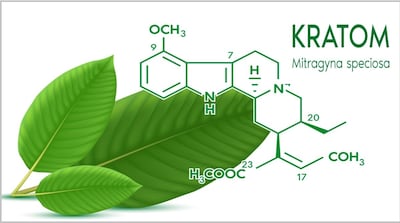
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
OTC erectile dysfunction treatment Eroxon has made it to the Nordics through local distributor Navamedic, which has reported strong consumer uptake.
A round-up of the latest global health and wellness moves: Nestlé and Dr Reddy's name JV head; Be-sup hires comms and regulatory director; Ascendis Health appoints interim CFO.
In this installment of HBW Insight’s “Inside Regulatory Affairs” series, we hear from Sharee Crumbey and Monica Sharda, regulatory affairs specialists at The Honest Company, about the mounting challenges and rewards of the job.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to CHPA’s Mike Bailey, senior vice president of regulatory and scientific affairs; and regulatory and scientific affairs VPs Marcia Howard and Jay Sirois.
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
Germany's Alzchem failed to convince the UK's health claims committee that daily creatine supplementation can contribute to improved cognitive function.
The FTC says its final rule banning fake consumer reviews and testimonials will help to restore its depleted toolbox for obtaining civil penalties. The agency maintains the final rule’s benefits will greatly outweigh costs for honest companies increasingly set back by bad actors in the space.
WishGarden Herbs marks 45 years with line extensions; Obesity doctor develops SoWell supplements for GLP-1 patients; Vital Proteins makes sustainability vital in packaging; and interior decoration and rent awards in vitaminwater campaign.
A round-up of the latest moves in Europe's consumer health industry: Galenica names head of new Products & Home Care business unit; Haleon appoints general counsel; Alliance Pharma eliminates COO role.
OTC sales edged up at Italy's Recordati in the first half of 2024 as higher demand for GI products and supplements offset a weaker cold & flu season.
Herbalife adds distributors and training as sales slow; Amphastar bullish on Primatene Mist $100M full-year target; and ChromaDex net sales up 12%.
OTC sales edged up at Italy's Recordati in the first half of 2024 as higher demand for GI products and supplements offset a weaker cold & flu season.
Avista VC acquires OTC CMO Trillium; FDA deputy commissioner Jones to open CRN conference; AG1 founder steps down, COO moves up to CEO; ChromaDex hires general counsel; Ajinomoto, Shiru partner to develop sweet proteins; and change at ingredient provider LBB helm.
ADVERTISEMENT