Durable Medical Equipment
The US FDA has published draft guidance for predetermined change control plans for medical devices along with recommendations for sponsors including them in marketing submissions to the agency.
Medtronic and Abbott announced partnership to integrate Abbott’s CGM sensor with Medtronic’s insulin pump, which marks a major departure from Medtronic’s former “closed-system” approach. Medtronic also announced FDA clearance of its Simplera CGM, paving the way for its use in smartpens.
Philips has filed a lawsuit against a Pennsylvania lab it hired to analyze sound abatement foam that prompted widespread recalls of its CPAP machines. Philips alleges PSN Labs grossly overestimated the risk to patients, which led Philips to initiate a larger recall than it otherwise would have.
While the National Electrical Manufacturers Association supports the Biden Administration’s plan to impose tariffs on a range of Chinese goods coming into the US, it also supports holding off on their implantation.
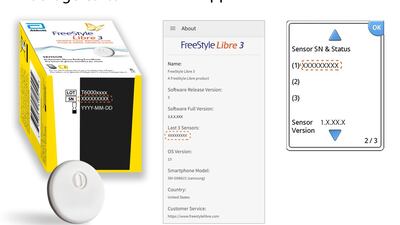
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
A New York district court ordered the asset freeze after finding that the owners of H&H Wholesale Services had concealed or gambled away money owed to Abbott. The company was ordered to pay Abbott $33m in 2023 for distributing test strips authorized for sale only outside the US.
The US FDA has issued two new warning letters to Chinese syringe makers Jiangsu Shenli and Jiangsu Caina following inspections of their facilities. The letters are the latest in what has been an ongoing investigation into these devices.
Last month, the US FDA increased the number of injuries and deaths initially reported in a March recall from Philips, which the company disputed. The FDA now says its adjusted numbers were in error.
The US FDA is reporting dozens more additional deaths associated with a May recall of Philips ventilators than initially reported. The company says it stands by its original number of seven and has reached out to the FDA.
Cutting edge technology could significantly enhance operating room efficiency, according to a new report from Medtronic. The problem, however, is that most ORs don’t have it.
Teleflex is recalling thousands of intra-aortic balloon catheter kits that are used during cardiac procedures and to treat complications from heart failure. This is the latest in a series of recalls from Teleflex and its subsidiary Arrow International.
A new round of tariffs imposed by the Biden administration on various Chinese goods, including medical devices, points to a broader shift in US strategy for strengthening supply chains and ensuring Americans have reliable access to safe products, according to analysts who spoke to Medtech Insight about the tariffs. While fueled by the pandemic, the momentum pushing this change in trade policy has been growing for some time.
The US FDA has issued a final rule allowing the agency to destroy some medical devices that have been refused entry into the US. The rule takes effect 1 July.
The US Food and Drug Administration released five warning letters last month, showing an ongoing focus on premarket clearance and the quality system regulations compliance.
The US FDA has issued a final rule allowing the agency to destroy some medical devices that have been refused entry into the US. The rule takes effect 1 July.
Abbott has issued a recall of several hundred HeartMate 3 pumps used to support patients with heart failure after reports of blood leakage and air entering the devices.
Medical products imported from China are among a broad range of items that will be subject to higher tariffs under new federal guidelines. The plan will raise tariffs on some forms of personal protective equipment (PPE) to 25%; additionally, tariffs on syringes and needles will be set at 50%.
This week, Philips Respironics reached a $1.1b settlement affecting CPAP and other breathing devices. Toku announced it received US FDA breakthrough device designation for its MyKidneyAI technology. This May, the FDA will hold its REdI conference focusing on innovation in medical product development and hold another townhall focusing on considerations for selecting a sterilization modality.
Kids ages 5 and up now can benefit from Cochlear’s Osia implant and sound processor, indicated for hearing loss, mixed hearing loss and single-sided sensorineural deafness.
A consent decree agreed to in January between Royal Philips and the US government is now official. The decree stems from a recall in 2021 of millions of the company’s sleep therapy and breathing devices due to risks posed by the sound abatement foam inside the machines.
ADVERTISEMENT