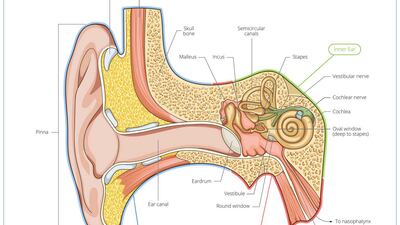
Ear
Finding the right way to measure outcomes is just one of the difficulties involved in conducting clinical trials of potential new therapies for loss of hearing, which can have a number of different causes.
A bipartisan bill now in the US Senate seeks to change the classification of implantable hearing aid devices to allow Medicare to reimburse for the devices, potentially expanding access for many Americans who require them.
More than a year after the FDA created a new rule allowing hearing aids to be sold over the counter and directly to consumers, a government report finds the category has not yet made the impact many were expecting.
More than a year after the FDA created a new rule allowing hearing aids to be sold over the counter and directly to consumers, a government report finds the category has not yet made the impact many were expecting.
The US biotech’s DB-OTO has made headlines worldwide after a baby with profound genetic deafness experienced improved hearing to normal levels within 24 weeks after a single intracochlear injection.
Norgine will market Pedmark, indicated to reduce risk of chemotherapy-related hearing loss in pediatric cancer, in the EU and UK. It will seek additional approvals in Australia and New Zealand.
In this first of a two-part roundup from CES, Medtech Insight tunes into two companies that developed assistive hearing devices, OrCam Hear and Concha Labs, and three women’s health companies focusing on menopausal symptoms and an app to track the “baby-making” journey.
Two executives of Israeli-based medtech companies say the Hamas-Israeli war is having an impact on their companies, with employees being called up as reservists and others needing accommodations. It also has galvanized companies to join forces to support health and humanitarian needs during the crisis.
A recent study published in the Journal of the American Medical Association compared the quality of OTC hearing aids and professional grade devices in treating mild to moderate hearing loss.
An innovative genetic test that can detect which babies are likely to develop deafness if treated with a common antibiotic is set to receive conditional funding in the UK under NICE’s rapid evaluation pilot scheme.
Listen up! In this episode of Medtech Monthly, Eargo CEO Christian Gormsen discusses the newly created OTC category for hearing aids.
The German biotech plans to prevent chemotherapy-induced hearing loss and treat chronic hearing loss by targeting a potassium channel in the ear’s sensory hair cells as it tackles a widespread but side-lined area.
With the planned $487m acquisition of sensorineural-focused Akouos, Lilly makes its second gene therapy M&A play in two years, on top of partnering/financing activity in the space.
A year after the US Food and Drug Administration proposed a new category for over-the-counter hearing aids, Americans can now buy them without a prescription.
US consumers have OTC access a year after FDA proposed category. While OTC category is limited to devices designed for mild to moderate hearing loss and not more severe hearing impairment, it covers vast majority of consumers with hearing loss.
After requirement from Congress and push from President Biden, FDA establishes regulatory category for hearing aids to allow US consumers with mild to moderate hearing loss to purchase the devices without a prescription or prior exam.
The US FDA has finally established a new regulatory category for hearing aids that will allow Americans with mild to moderate hearing loss to purchase the devices directly over the counter without a prescription or prior exam.
The CEO of direct-to-consumer hearing aid manufacturer Eargo says an OTC category for hearing aids would improve the quality of life for many Americans with hearing loss.
Some of the top hearing aid firms engaged in fraudulent messaging to undercut the sale of hearing aids directly to consumers, according to a bipartisan Senate investigation.
The company, which denies the allegations, allegedly sold hearing aids to federal employees and billed a government insurance plan without the required hearing assessment.
ADVERTISEMENT