Ear, Nose & Throat
At the recent Precision Med-Tri Con conference, laboratory experts traded views on the expansion of at-home testing for disease diagnosis and personalized health insights. While strong consumer demand spells opportunity, there are significant concerns about the accuracy and reliability of home-testing platforms, misuse, accessibility, and lack of health literacy.
Kids ages 5 and up now can benefit from Cochlear’s Osia implant and sound processor, indicated for hearing loss, mixed hearing loss and single-sided sensorineural deafness.
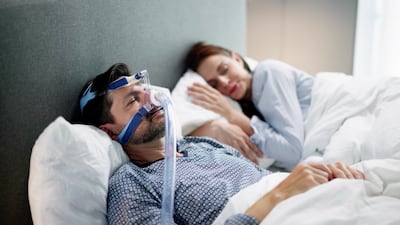
Topline results from two studies in obstructive sleep apnea among obese adults showed efficacy crossing the 50% threshold that physicians have called clinically meaningful.
In this second roundup from CES, Medtech Insight shines the light on Proto Hologram’s many use cases in health care, Intuition Robotics’ ElliQ robot to help the elderly feel less lonely, MIT AgeLab’s Aging Suit, Siemens/Unlimited Tomorrow’s partnership on a bionic prosthetic arm, and Earable Neuroscience’s Frenz Brainband to promote better sleep.
Integra will pay $275m in cash plus $5m more in potential milestone incentive payments to add Acclarent’s ear, nose, and throat surgery devices to its existing neurosurgery portfolio.
A very early success with a gene therapy for a rare form of deafness puts Regeneron ahead of Lilly and Sensorion.
Two executives of Israeli-based medtech companies say the Hamas-Israeli war is having an impact on their companies, with employees being called up as reservists and others needing accommodations. It also has galvanized companies to join forces to support health and humanitarian needs during the crisis.
Medline Industries has issued a recall of some of its saline solution products for being non-sterile. The US FDA has designated the recall class I.
Medtech Insight recently sat down with TytoCare’s chief commercial officer Tamir Gotfried to discuss plans for driving virtual care in the home, and engage physicians and consumers, at a time when pandemic-era state-level telehealth flexibilities continue to evolve.
The US FDA has cleared a new endoscopy system from Olympus along with two compatible gastrointestinal endoscopes for the diagnosis and treatment of a range of GI issues.
A number of new drugs have taken a major step towards being approved for use in the EU after receiving the nod from the European Medicines Agency’s human medicines committee, the CHMP. Meanwhile, the marketing applications for three drugs have been withdrawn.
The European Medicines Agency’s human medicines committee, the CHMP, is this week deciding whether to back marketing approval for eight new drugs and a coronavirus vaccine.
The ENT specialist reportedly purchased only 36 single-use sinus devices but performed at least 1,400 procedures. She could land up to 40 years in prison after being convicted on 20 counts including device adulteration, conspiracy, and fabricating medical records.
When is a borderline drug/device product a medical device? Only when the manufacturer can prove that is definitely not a medicine, according to a new EU Court Of Justice ruling. Expert medtech regulatory lawyer Erik Vollebregt outlines his main criticisms of this judgment.
In this second roundup of innovative health solutions showcased at CES 2023, Medtech Insight highlights SK Biopharmaceuticals’ new wearables in development for early detection of seizures, Dassault Systèmes’ simulated 3D heart, and Lighthouse Tech’s eyewear to help the visually impaired detect obstacles.
After successive CRLs in 2020 and 2021, the drug gained a broad label for hearing loss in children treated with cisplatin for localized, non-metastatic solid tumors.
The US FDA has received more reports of adverse outcomes, including deaths, from the breakdown of foam in many sleep apnea devices from Philips.
The FDA recently cleared the TruDi Shaver Blade, an electromagnetically navigated blade for the incision and removal of soft and hard tissue or bone in ear, nose, throat, and maxillofacial surgery, as well as head and neck and skull-base surgery.
With positive Phase III data for its Xhance (fluticasone propionate) nasal spray in chronic sinusitis, Optinose believes it is poised to expand the target market for the drug 10-fold.
Medtech Insight talked with Moshe Shoham about the innovative technologies behind surgical robotics companies Microbot Medical, XACT Robotics, Tamar Robotics and ForSight Robotics, which were all spun out of his laboratory in Israel.
ADVERTISEMENT