Europe
Steffen Wagner has taken over as Stada’s head of Germany, adding to his existing responsibilities in Europe, after Eelco Ockers stepped down from the role. Ockers’ departure comes as German generics giant Stada restructures its business to bring its branded and consumer health interests into closer alignment as part of a more streamlined model.
In line with its plan to expand into the Nordics, CNS specialist Neuraxpharm has established a new business unit in Stockholm and has recruited Viatris’ Magnus Wassén to lead it as general manager. The company is expecting Neuraxpharm Sweden to provide an immediate sales platform for Buccolam.
Formycon and partner Bioeq have offered further details on timing for the submission of their FYB201 proposed ranibizumab biosimilar version of Lucentis in Europe, the US and elsewhere.
After partnering with companies like Purna Female Healthcare and AFT Pharmaceuticals, Belgian value-added medicines specialist Hyloris has announced plans to add three new products to its pipeline in the coming months with a goal of having 14 commercial products by 2024.
Neuraxpharm will gain a platform in Ireland after striking a deal to acquire local distribution specialist Medinutrix.
Accord Healthcare has struck a European licensing deal with Orexo for its Zubsolv opioid-dependence treatment, building on a CNS business that also includes a recent distribution agreement with Molteni Farmaceutici for Sixmo.
Nidda Healthcare, the investment vehicle controlled by Bain Capital and Cinven, has secured a “timely” squeeze-out of minority shareholders in Stada through settlement terms that include a one-time payment of €0.10 per outstanding share for the remaining shareholders.
Medicines for Europe has highlighted the need for an improved digital strategy across the EU in order to improve patient information and access to medicines, among other benefits like reducing the risk of supply disruptions, especially in the context of the current pandemic.
<p>Executive Summary</p> <p>A list of EU biosimilar filings, CHMP opinions and EU marketing authorizations, including details of the biosimilar company, the brand name/INN, indication(s), the reference product/company, and the date and type of event.</p>
Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.
Technical discussions on the EU pharmaceutical legislative reform are underway at the Council of the EU.
As the reform of the EU pharmaceutical legislation progresses through the legislative process, the Pinks Sheet offers an infographic highlighting some of the key changes being proposed that will reshape the way drug companies run their business.
<p>Executive Summary<p> <p>The Pink Sheet's list of EU centralized approvals of new active substances has been updated to add six new products, incl/ding Ixchiq, Valneva's chikungunya virus vaccine</p>
<p>Executive Summary</p> <p>Zanidatamab, Jazz and BeiGene’s investigational treatment for biliary tract cancer, is among the latest products that have been filed for review by the European Medicines Agency for potential EU marketing approval.</p>
<p>Executive Summary</p> This is an update of recommendations from the European Medicines Agency's Committee for Medicinal Products for Human Use on the authorization of new medicines in the EU, and updates on EU marketing authorization changes recommended by the CHMP.
<p>Executive Summary</p> <p>A list of EU biosimilar filings, CHMP opinions and EU marketing authorizations, including details of the biosimilar company, the brand name/INN, indication(s), the reference product/company, and the date and type of event.</p>
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.
A total of 748 key medicines are now affected by the four-month stock requirement, compared with 422 in 2021.
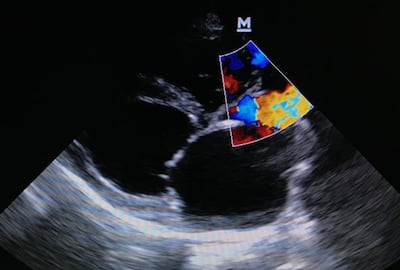
TRiCares announced first implantation of the Topaz transfemoral tricuspid heart valve replacement system as part of the company’s EU pivotal study. If all goes to plan, the device will compete with Edwards’ Evoque system. The announcement follows the company’s $50m series D funding raise in July.
UK-based Biocomposites will begin selling in the UK its next-generation osteoinductive bone graft substitute, NanoBone, which came with its acquisition of Artoss GmbH in June 2023. Meanwhile, the company has purchased remaining shares in the manufacturers of SYNICEM and Subiton antibiotic bone cements and preformed antibiotic-loaded spacers.
ADVERTISEMENT