Georgia
Country
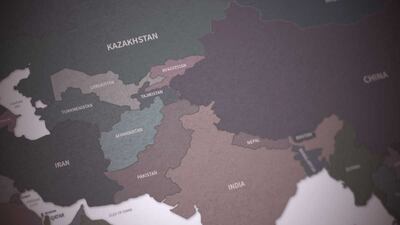
Russia and Ukraine had been key players in the pharma markets of Central Asian and surrounding countries before the launch of the Russian invasion into its neighbor on 24 February. Because of this, both countries are poised to give ground in this growing regional market.
The first of around 260 patients for a clinical trial comparing its PB006 natalizumab biosimilar candidate to Biogen’s Tysabri reference brand have been recruited by Polpharma Biologics, which has just added Karsten Roth and Alexandra Moulson to its team.
Stada will pay $660m for a basket of 20 brands from Takeda to expand its consumer health portfolio in Russia and a number of strategically important CIS markets.
Reporting double-digit growth in the first half of 2019, Krka says it has mined marketing opportunities in new eastern European markets by introducing its established treatments for cardiovascular diseases
When Scrip first wrote about Zika virus (ZIKV) less than two weeks ago there was no record of any clinical development ongoing for a treatment or vaccine against the mosquito-transmitted disease, let alone any available therapies.
With the theft of American companies' trade secrets increasingly on the rise – including those belonging to biopharmaceutical firms – lawmakers last week said more needs to be done to ensure there are stronger legal protections in place to secure that commercially valuable information.
While Bristol-Myers Squibb & Co.'s programmed death-1 (PD-1) immune checkpoint inhibitor Opdivo (nivolumab) is well ahead of Merck & Co.'s competing drug Keytruda (pembrolizumab) in FDA approved indications, it's the latter drug that's likely going to stick in the minds of the American public – at least for the time being.
Asterias BioTherapeutics, Inc. has appointed Georgia Erbez chief financial officer (CFO) – effective Nov. 9. Erbez has served as financial consultant to various biotech companies and most recently was CFO, secretary, and treasurer of Raptor Pharmaceutical Corp. Prior to this, she was managing director, healthcare investment banking at Collins Stewart, a senior level investment banker at Beal Advisors, Jeffries & Company, Inc. and Cowen and Company
The FDA's Arthritis Advisory Committee (AAC) on Oct. 23 in a 10-4 vote recommended approval of lesinurad, an experimental drug from AstraZeneca plc and its US unit Ardea Biosciences Inc., as a treatment in combination with a xanthine oxidase inhibitor (XOI), such as allopurinol or febuxostat, for hyperuricemia associated with gout in adults.
It's no surprise the US Centers for Disease Control and Prevention (CDC) was not eager to make public a scathing report from a panel of outside expert advisers that condemned the agency's laboratory safety practices – declaring it "is on the way to losing credibility" after a series of near-calamities involving anthrax, the H5N1 influenza virus and Ebola.
The outgoing head of the FDA on 10 March admitted there are 172 guidance documents that are in draft form that need to be finalized, revised or withdrawn – with one remaining in limbo since 1988.
Valeant Pharmaceuticals pulled Dendreon out of bankruptcy by acquiring the troubled prostate cancer immunotherapy company, and Johnson & Johnson's Janssen Biotech subsidiary pumped much-needed cash into Geron with a drug development partnership, but because of those deals 95 people at Dendreon and Geron are losing their jobs.
Before Southern California biotech startup BCN Biosciences became engaged with a new program at the National Institutes of Health (NIH), the firm thought it had one indication for its experimental small molecule BCN057 (YEL002) – radiation mitigation.
A majority of an FDA panel of advisers on 16 October recommended retaining the black-box warning on the labeling of Pfizer's anti-smoking aid Chantix (varenicline), which alerts prescribers and patients about the risk of serious neuropsychiatric events, and reassessing it once results are available from an ongoing postmarketing study, expected in August 2016.
Catalyst Pharmaceuticals plans to initiate a rolling new drug application (NDA) for Firdapse in early 2015, pending pre-NDA discussions with the US FDA, based on positive Phase III results in the treatment of Lambert-Eaton myasthenic syndrome (LEMS).
Boehringer Ingelheim easily convinced an FDA panel of outside advisers on 14 August to back approval of the firm's latest chronic obstructive pulmonary disease (COPD) drug Spiriva Respimat (tiotropium bromide), with the committee voting 10-3 at a meeting that could almost be described as sleepy.
Biota Pharmaceuticals will no longer develop laninamivir octanoate (LANI) on its own following the inhaled flu treatment's failure in a 639-patient Phase II clinical trial that lost US government backing in May.
After the markets closed on 20 June, the FDA gave the good news to Cubist Pharmaceuticals that its antibiotic Sivextro (tedizolid phosphate) gained the agency's approval as a treatment for acute bacterial skin and skin structure infections (ABSSSI) caused by Gram-positive bacteria, such as Staphylococcus aureus, including methicillin-resistant (MRSA) and methicillin-susceptible strains, various Streptococcus species and Enterococcus faecalis.
Health and Human Services (HHS) Secretary Kathleen Sebelius took one step closer to exiting her position in running the agency after the Senate Finance Committee voted 21-3 in favor of backing the health chief's replacement, Sylvia Mathews Burwell.
Ernst & Young's recent report Progressions: Navigating the payer landscape noted the lack of trust that health care payers have for pharmaceutical companies, but if you look closely EY's survey of payers and industry revealed opportunities for biotechnology firms to help big pharma engage with private insurers and government agencies that cover drug costs (scripintelligence.com, 7 May 2014).
ADVERTISEMENT