Government Payers
The US government continued its focus on False Claims Act enforcement and collected funds totaling more than $1bn in the first of this year alone – $155m of which was medtech-related.
The Medical Devices Information System (MeDevIS) platform, launched by the WHO this week, consolidates information on 2,301 device types and streamlines device nomenclature to support informed decision-making by governments, regulators, payers, and healthcare providers.
The efficacy of Moderna’s jab appears to fade faster than its rivals, while experts were surprised by an FDA panel’s recommendation to narrow the use of all three RSV vaccines.
The pathway, which would be separate from breakthrough device reimbursement, would allow new AI technology to be reimbursed based on “clinical value and public stakeholder engagement.”
Seth Berkley, epidemiologist and former CEO of Gavi, the vaccine alliance, has emphasized the importance of making “investments in peacetime” to prepare for pandemic threats and called for a “mindset change” among global governments.
BioNTech and the UK government's alliance to bring personalized cancer immunotherapy to patients is making progress, but the National Health Service has to show it can deliver on clinical trials recruitment.
The fourth court loss for pharma in industry's attempts to kill Medicare’s drug price negotiation program adds to the list of reasons courts have rejected legal challenges to the Inflation Reduction Act.
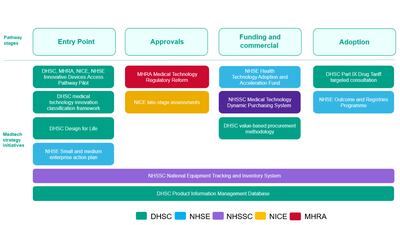
Fourteen months on from the release of its inaugural medtech strategy, the UK MedTech Directorate has laid firm foundations and reports progress on initiatives aimed at improving technology adoption. A schedule of ambitious future timelines has also been published.
Sandoz argued against paying higher rebates for two of its drugs via the Centers for Medicare and Medicaid Services’ Medicaid drug rebate program in a US court six years after the suggestion was first made.
Sandoz has fired a defensive shot in a US court against the potential that it may have to fork up higher rebates for two of its drugs via the Centers for Medicare & Medicaid Services’ Medicaid drug rebate program – six years after the suggestion was first made.
The journey to Net Zero, described as ‘the defining issue of our time,’ will get harder in the coming decade. Failure to keep up the pressure will result in more long-term health conditions, increasing deaths and higher costs. The UK NHS is a Net Zero exemplar globally, but without a systemic approach, its compliance efforts could stutter.
A new report from the US Government Accountability Office found that the Veterans Health Administration does not track implanted devices at an individual-patient level, which could make it harder to communicate important safety information in the event of a recall.
President’s State of the Union address doubled down on his desire to expand on the IRA. While the agenda poses no near-term threat, it will keep pharma on its toes as industry juggles implementation of the new Medicare prices with fighting the IRA in court and trying to reform the original law.
Among the provisions in a newly tabled bill seeking to introduce a national pharmacare system in Canada is a bulk purchasing strategy for prescription drugs that could help lower costs.
The AAM’s annual Access! 2024 conference brought its much-enjoyed CEOs Unplugged panel to Tampa again for another engaging discussion on critical issues, challenges and opportunities for generics and biosimilars in the US.
The well-known businessman and TV personality told the White House that the US needs to ‘stop doing business with the big three PBMs,’ helping to fight the drug industry’s battle against the middlemen with a friendlier face not associated directly with the brand industry.
The government collected $2.68bn from settled False Claims Act cases last year, the US Department of Justice said. Another 12 device-related cases were announced in February alone.
South Korea’s healthcare services face both longer-term systemic challenges and short-term disruption from ongoing doctor walkouts over government plans to boost medical trainees. Meanwhile, the local pharma industry is calling for new measures to support the supply of essential drugs.
PBM reform isn’t expected to ride along with the next government funding packages, which could push any prospect of legislative reform closer to 2025. Meanwhile FTC says it is facing difficulty getting all the information for its investigation.
The Medicare draft guidance seems designed to ensure seniors don’t miss out on the IRA benefit.
ADVERTISEMENT