Ingredients
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
In this episode of the Over the Counter podcast, HBW Insight speaks to Kerry's RDA senior manager of immune and joint health, Sonja Nodland, about the opportunity for innovation that postbiotics represent for the consumer health industry.
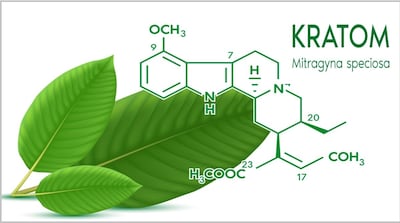
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Height of plaintiff attorney’s argument to present evidence which would prompt speculation by a jury was request to parse research by Kenvue’s lead expert, who coordinated the International Consensus Statement on ADHD by the World Federation of ADHD, where he’s president.
Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”
David Ball moves from Bayer’s North America business to be Perrigo’s first chief brand and digital officer. He joins CEO who also moved to Perrigo from Bayer with decades of branded product experience.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
Germany's Alzchem failed to convince the UK's health claims committee that daily creatine supplementation can contribute to improved cognitive function.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
Herbalife adds distributors and training as sales slow; Amphastar bullish on Primatene Mist $100M full-year target; and ChromaDex net sales up 12%.
With monographs@FDA portal down, agency moves to NextGen and extends comment period through 27 September for first OTC monograph change it proposed using its overhauled program other than 32 monographs finalizations required in 2020 legislation authorizing overhaul.
Putative class-action complaint alleging false advertising for Tylenol Rapid Release gelcaps recently dismissed on J&J Consumer’s motion arguing that federal preemption prohibits requiring different or additional labeling for drugs available under FDA OTC monograph.
A two decade-long journey seeking US Food and Drug Administration approval of sunscreen UV filter BEMT is nearing its end, according to DSM-Firmenich’s Carl D’Ruiz. DSM-Firmenich’s experience speaks to the complexities and costs that sponsors face in the US in bringing new active ingredients to the OTC sunscreen products category.
Efforts to unite as an industry to provide the US Food and Drug Administration with data to fill safety gaps for “chemical” UV filters have fizzled as the agency insists on animal-test requirements. Without a collaborative effort to share resources and costs, the long-marketed sunscreen actives may not receive the investment needed to demonstrate GRASE, and the US market may decay further in comparison with the diversity of modern sun-protection options available to consumers abroad.
The firm’s development of VagiBiom prebiotic and probiotic supplements and suppositories and Vee feminine health tracking app emanated from founder Bobban Subhadra’s interest in the health potential of the human microbiome and research in the fields of microbiology and immunology.
Germany's Expert Committee on Prescription recommends the Rx-to-OTC switch of an azelastine and fluticasone propionate combination nasal spray, the second time around. Low-cost smoking cessation drug cytisine, on the other hand, is denied - also the second time it has appeared before the committee.
Haleon has begun reformulating phenylephrine-containing oral decongestants marketed in the US as the company looks to get ahead of an anticipated FDA decision on the ingredient's use in OTC cough and cold products. The UK-based consumer health manufacturer revealed its plan as it reported organic sales up 3.5% in the first half of 2024.
Critics expect FDA already has reached conclusion about compelling making kratom a controlled substance. “Their conclusion seems to be pretty well preordained,” says NPA CEO Daniel Fabricant.
UK microbiome specialist ProBiotix Health has combined its patented LPLDL probiotics strain with thiamine (vitamin B1) to create an convenient supplement ingredient for lowering cholesterol and supporting overall cardiometabolic health.
ADVERTISEMENT