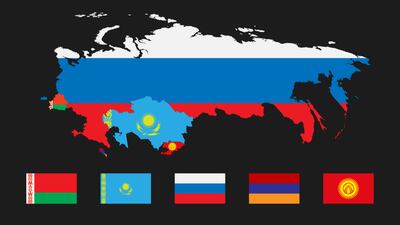
Kazakhstan
Country
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have since stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
After a five-year regulatory transition, access to the Eurasian Economic Union market for new medtech products must now take place via the EAEU system only. But having got off to a slow start, further system transitional measures are now under discussion.
The Eurasian Union’s medical device regulatory system, many years in the planning, becomes mandatory in January 2022. But new amendments posted in late summer allow for national registrations to remain valid after the deadline. Moscow-based consultancy RegMT explains the background, and the likely path ahead.
There are just 4.5 months left until the new harmonized Eurasian Economic Union medtech system becomes mandatory, signifying major changes in regional market access. EAEU member Russia is meanwhile strengthening its post-market medtech controls.
The EMA has issued updated safety advice on AstraZeneca’s COVID-19 vaccine, Vaxzevria, Valneva has decided to pursue vaccine supply deals with individual member states after a falling out with the European Commission, and Kazakhstan has begun administering the first doses of its domestically produced vaccine, QazVac.
Kazakhstan’s pharmaceutical market showed positive dynamics in 2020. In the first 9 months of last year, market size for finished pharmaceutical products grew to KZT460bn($1bn), up 22% year-on-year.
The Medicines Patent Pool is giving interested parties until 18 December to submit license applications for the HIV treatment dolutegravir in four middle-income countries, following announcement of the agreement last month.
Dr Reddy's is poised to expand its OTC offering in Russia and certain CIS countries by snapping up a basket of allergy brands from fellow Indian firm Glenmark.
Reporting double-digit growth in the first half of 2019, Krka says it has mined marketing opportunities in new eastern European markets by introducing its established treatments for cardiovascular diseases
Russia and Kazakhstan, two prominent members of the Eurasian Economic Union, are continuing to build national medtech regulatory infrastructures, in the shadow of the forthcoming harmonized EAEU system, of which a postponement beyond 2021 has not been ruled out.
British probiotic specialist OptiBiotix Health has struck a three-year distribution deal for its CholBiome supplement in Russia and Kazakhstan.
The new regulatory framework in the Eurasian Economic Union is now in place and the first drug approval applications have been filed under the mutual recognition system. Applications are expected to rise significantly in 2019 once IT systems are fully in place and more experience has been gained.
Various economies of the Commonwealth of Independent States (CIS) and the Eurasian Economic Union (EAEU) are pressing ahead with medtech regulatory changes nationally and/or regionally, which can only be good for the delivery of quality care over the long term. But a question mark still hangs over the official start date of the EAEU medtech regulatory system; a formal postponement may be decided soon.
It might seem that the Eurasian Economic Union (EAEU) agreement on medical device regulation is nothing but administrative issues and slow progress, but it should deliver benefits to all stakeholders after launch, currently slated for 2022. but manufacturers need to be mindful of the member states' strengths and weaknesses when selecting their reference member state.
The transition deadline for adoption of Eurasian Economic Union (EAEU) medtech principles across the five member states remains the end of 2021, but vital elements are still not resolved, giving rise, for the first time, to notions of a delay in adoption. This would be the pragmatic course, local market experts believe, but for now, efforts are aimed at completing the system on time.
Saudi Arabia has started the application process to join the Pharmaceutical Inspection Cooperation Scheme, while bids from Belarus, Chile, Brazil, Iran, Mexico, Turkey and other countries are wending their way through the PIC/S application procedure.
The much-anticipated Eurasian Economic Union (EAEU) was originally targeted to come into effect at the start of 2016, but more than year later, the five-member trading bloc has yet to be ratified by all nations. The Russian medtech industry is impatient for progress, but IMEDA's Sergey Kolosov sees some promising signs in recent developments.
More building blocks of the Eurasian Economic Union’s common medical technology regulatory system are falling into place – but are developments taking place fast enough for an early 2017 launch?
The Eurasian Economic Union's system for regulating medtech products is dropping into place, but too slowly as some see it, and still with many questions remaining over the content. However, it remains a fixture for the future, and device companies serving the region need to be prepared.
ADVERTISEMENT