Legislation
With the US BIOSECURE Act waiting for a Senate vote, there are signs it may be prompting some Chinese firms to look at their operations. In the meantime, two legal experts in China suggest a range of coping strategies for companies that may be deemed "of concern."
With the US BIOSECURE Act waiting for a Senate vote, there are signs it may be prompting some Chinese firms to look at their operations. In the meantime, two legal experts in China suggest a range of coping strategies for companies that may be deemed "of concern."
Avoiding disaster at the beginning of the pandemic and offering a pro-active supply chain blueprint should position the generics industry well for an onshoring debate in Congress, but a look back at March 2020 is a reminder that even small policy shifts can have a big impact.
Technical discussions on the EU pharmaceutical legislative reform are underway at the Council of the EU.
Multi-cancer diagnostics can help get oncology patients the treatment they need more quickly, but lack of reimbursement has kept such tests out of reach for many patients. Bills providing coverage have passed or are under consideration in more than half of the states and have been introduced in both houses of US Congress.
An employee of Astellas in China first detained 17 months ago on suspicion of espionage has been formally indicted and now faces trial.
Congressional Budget Office responses to questions from US lawmakers on pharmacy benefit managers could add to the momentum for PBM reforms to go further than the current leading proposals.
Letter to US FDA by a bipartisan group of House members cites clinical trials sponsored by Eli Lilly and Pfizer as examples of a widespread industry practice. But any legislation to curtail such practices is not expected to be part of the BIOSECURE Act.
A government report requested by US lawmakers Debbie Dingell and Anna Eshoo to review the FDA’s postmarket surveillance of medical devices stresses that strengthening the system is critical to addressing adverse events linked to devices after they hit the market.
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
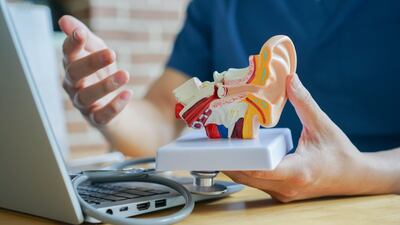
A bipartisan bill now in the US Senate seeks to change the classification of implantable hearing aid devices to allow Medicare to reimburse for the devices, potentially expanding access for many Americans who require them.
With monographs@FDA portal down, agency moves to NextGen and extends comment period through 27 September for first OTC monograph change it proposed using its overhauled program other than 32 monographs finalizations required in 2020 legislation authorizing overhaul.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”
Another House hearing again showcases the bipartisan desire to enact reforms to the US pharmacy benefit management sector. Legislators also sound ready to push for more significant actions than “transparency” measures would promise, but that means waiting until 2025.
While the National Electrical Manufacturers Association supports the Biden Administration’s plan to impose tariffs on a range of Chinese goods coming into the US, it also supports holding off on their implantation.
Just under a third of UK parents who smoke or have smoked in the past believe there isn’t enough support to quit, and nearly a quarter don’t know where to go for support, Kenvue reports, based on recent surveys. In response, the firm launches the “Smokefree Families” initiative to help the 1.8m households in England with children and at least one smoker to become smoke free.
CRN appreciates Durbin’s “commitment to greater transparency in the dietary supplement marketplace,” but “cannot endorse it in its current form”; AHPA asks for better option; and NPA remains opposed.
In June, the US Supreme Court reversed the Chevron doctrine, a long-standing precedent requiring courts to defer to regulatory agencies when statutory language was ambiguous. But will that decision prevent the FDA’s final rule on laboratory developed tests from taking effect? A legal expert weighs in.
While a recent FTC probe revealed PBM practices that block access to generics and biosimilars, a new report by the US House Committee shows that the three big PBMs may seek a safe harbor in foreign countries to avoid scrutiny.
Marketing authorizations for OTC medicines could be rejected if their environmental risk assessments do not meet new requirements proposed within the EU pharma legislation revision. HBW Insight speaks to regulatory law experts Tine Carmeliet and Eline D'Joos to find out what you need to know about the new rules.
ADVERTISEMENT