Manufacturing
Avoiding disaster at the beginning of the pandemic and offering a pro-active supply chain blueprint should position the generics industry well for an onshoring debate in Congress, but a look back at March 2020 is a reminder that even small policy shifts can have a big impact.
After US President Joe Biden launched a review of the country’s API supply chain, the task force has reported back with key vulnerabilities that contribute to drug shortages and supply risks during a global public health emergency. According to the report, the lack of geographic diversity and dependence on foreign nations and anti-competitive actions by foreign nations are key areas of concern.
Nichi-Iko says that remediation activities at its Toyama plant in the wake of a suspension over GMP violations mean that there may be delays to supply.
Biocon Biologics has announced the latest milestone in its integration of former partner Viatris’s biosimilars business, completing the move in the US and Canada “ahead of schedule.”
To tackle the increase in demand for remdesivir in India amidst the second wave of COVID-19, Gilead Sciences is helping local licensees to scale up production of the product, including by providing API supplies. The company is also donating at least 450,000 vials of the Veklury brand “to help address the immediate needs of Indian patients.”
To increase transparency and affordability, American billionaire and businessman Mark Cuban has launched a US generics venture under the name ‘the Mark Cuban Cost Plus Drugs Company.’ Cuban’s new company is hoping to introduce over 100 drugs by the end of 2021 and build a pharmaceutical factory of its own by 2022.
Teligent is pursuing an asset sale process to “maximize the value of the company” after entering into Chapter 11 bankruptcy proceedings in the US. The firm – which has also seen CEO Tim Sawyer resign – had been struggling of late with remediation issues to address a warning letter at its Buena manufacturing facility in New Jersey, as well as a recent recall.
A fresh executive order from US president Joe Biden calls for short-term and long-term study, broad consultation, and co-ordination with allies on the domestic supply chain.
Antibiotics specialist Centrient has struck a deal to acquire India’s Astral SteriTech in a move that will expand its portfolio to include sterile injectable finished-dose products.
The company will ramp up manufacturing of its Jynneos vaccine to meet demand in worst-affected African countries and stockpiling richer nations.
This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.
The agency’s plan for advanced manufacturing seeks more harmonization, while also seeking to codify internal practices with guidance and training.
With Sagent Pharmaceuticals operating in an increasingly competitive US injectables market, the company has recently been making moves to bolster its position in the wake of its separation last year from its former parent company, Japan’s Nichi Iko. Chief executive Vishy Chebrol talks to Generics Bulletin about the firm’s strategic goals.
The bispecific antibody is the latest example of a growing number of biologics receiving complete response letters related to third-party manufacturing facilities.
A US FDA analysis found that facility inspection issues were the fastest growing factor in the recent rise of complete response letters for biologics licensing applications, in part reflecting the limitations of single-product inspections at contract facilities manufacturing multiple products.
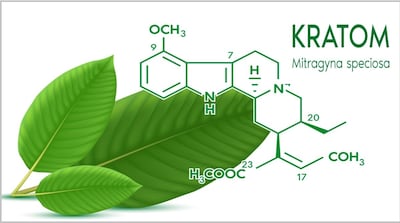
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Conflicted experts should be allowed to participate as nonvoting members and panels should take a benefit-risk vote on product-specific applications, the majority of respondents said in a survey conducted by 3D Communications.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
ADVERTISEMENT