Patient Monitoring
Edwards Lifesciences’ Critical Care business “invented the hemodynamic monitoring category, and its solutions are currently used in more than 10,000 hospitals globally to better understand the cardiovascular condition in real-time for critically ill patients, which helps improve outcomes,” says BD, which sees synergies and new innovation opportunities across the groups’ data sets and platforms. Acquired for $4.2bn cash, Critical Care generated more than $900m in revenue in 2023.
The US FDA has granted marketing authorization to NOWDiagnostics for the first at-home over-the-counter test to detect syphilis, which the government says is on the rise.
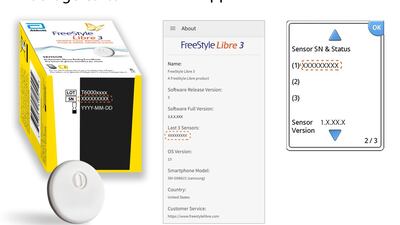
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
The FDA has published final guidance to assist developers of medical devices designed to treat opioid use disorder, along with considerations for sponsors of clinical studies to evaluate those devices.
The US Federal Trade Commission wants to clean up the FDA’s Orange Book by purging medical device patents that the commission says should not be in the listing. The FTC argues improper patents in the Orange Book block lower-cost generic equivalents from coming to market. Medtech Insight spoke to attorney Sara Koblitz about the FTC’s delisting push.
The US FDA is reporting dozens more additional deaths associated with a May recall of Philips ventilators than initially reported. The company says it stands by its original number of seven and has reached out to the FDA.
Cutting edge technology could significantly enhance operating room efficiency, according to a new report from Medtronic. The problem, however, is that most ORs don’t have it.
Teleflex is recalling thousands of intra-aortic balloon catheter kits that are used during cardiac procedures and to treat complications from heart failure. This is the latest in a series of recalls from Teleflex and its subsidiary Arrow International.
Record attendance at the 2024 MedTech Forum in Vienna heard industry leaders voice optimism grounded in realism about future developments for Europe’s medtech industry, markets and delivery of care to patients. In Vivo filtered out 16 trends to watch.
Stacey Churchwell, VP and general manager for cardiovascular diagnostics and services, discusses how AI has enabled Medtronic to “clean up the noise” and vastly reduce false alerts sent by its Reveal LINQ insertable cardiac monitor, saving clinics many hours of needless reviews, bolstering confidence in continuous monitoring, and alleviating liability concerns among health care providers.
The US FDA has granted 510(k) clearance to a California medical technology company for a brain monitoring device that detects seizures in patients with brain injury or trauma.
Exergen seeks a declaration from Massachusetts federal court that claims challenged by Baxter in the National Advertising Division forum regarding the accuracy of its thermometers do not constitute false or misleading advertising, if they are even advertising at all.
Exergen seeks a declaration from Massachusetts federal court that claims challenged by Baxter in the National Advertising Division forum regarding the accuracy of its thermometers do not constitute false or misleading advertising, if they are even advertising at all.
Dexcom launches its highly customizable One+ continuous glucose monitor in the UK as well as a state of type 2 report that suggests 63% of people living with type 2 diabetes have trouble managing their disease.
The designation makes the watch's AFib history feature the first digital health tool to be qualified under the US FDA’s Medical Device Development Tools program, meaning it can be used in research during the development of certain cardiac devices.
A novel robotic-assisted therapy could improve the lives of men with benign prostatic hyperplasia, real-world data presented at the annual meeting of the American Urological Association suggests.
This week, Philips Respironics reached a $1.1b settlement affecting CPAP and other breathing devices. Toku announced it received US FDA breakthrough device designation for its MyKidneyAI technology. This May, the FDA will hold its REdI conference focusing on innovation in medical product development and hold another townhall focusing on considerations for selecting a sterilization modality.
The US FDA has cleared a glucose monitoring system from Senseonics. The authorization of the implantable insulin delivery system creates a new pathway for like devices.
Vivalink will provide ECG wearable technology to monitor patients in RSRT’s Vibrant study, which is aimed at assessing autonomic dysfunction in children with Rett syndrome.
A study of diabetes rates across the US over four years reveals significant increases in the disease in many states. Tobias Oerum, diabetes advocate and cofounder of the company that conducted the study, discussed the data and some of the factors contributing to this troubling trend with Medtech Insight.
ADVERTISEMENT