Platform Technologies
The US precision medicine company hopes its drug response predictor technology will enhance responses to the chemotherapy Ixempra on the basis of promising data from a handful of breast cancer patients in a Phase II trial, marking a positive turn in its journey to date.
The US FDA has published draft guidance for predetermined change control plans for medical devices along with recommendations for sponsors including them in marketing submissions to the agency.
Emerging Company Profile: Backed by Flagship Pioneering, Empress deploys its Chemilogics platform to seek leads for small molecule therapies in inflammation, immunology, cancer and metabolic disease.
Halda will take its first RIPTAC molecule into a clinical trial in prostate cancer, offering a new small molecule modality in an indication where patients and doctors prefer oral drugs.
Major Japanese firm picks up US drug discovery platform company Jnana in apparent bid to strengthen discovery capabilities and build its mid-stage R&D presence in the renal area.
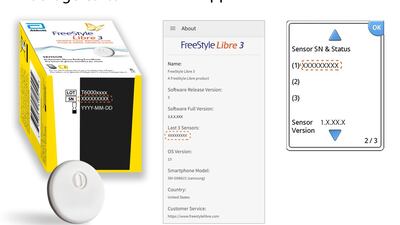
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
Many platform designation requests have been from sponsors eager to cite other sponsors’ products, but CBER Director Peter Marks said in an interview with the Pink Sheet his office likely is years away from accepting those applications.
Andrew Trister, Verily’s chief medical and scientific officer, discusses Verily’s newly launched Lightpath Metabolic solution, featuring GLP-1 prescription, AI and strengthened clinical support. Plus, the Google health tech spinout's plans for the Study Watch, the Alzheimer’s research landscape, and AI development and regulation in a new era of uncertainty.
Gain Therapeutics aims to offer a disease-modifying treatment for Parkinson’s patients with GCase mutations. The company’s promising data and platform face competition from notable rivals.
Andrew Trister, Verily’s chief medical and scientific officer, spoke with Medtech Insight at the HLTH Europe conference about Verily’s newly launched Lightpath Metabolic solution, featuring GLP-1 prescription, AI and strengthened clinical support. Trister also talked about plans for the Study Watch and offered views on the Alzheimer’s research landscape and AI development and regulation in a new era of uncertainty.
Cutting edge technology could significantly enhance operating room efficiency, according to a new report from Medtronic. The problem, however, is that most ORs don’t have it.
Deal snapshot: J&J decided to return global rights to platotamab, a Phase II-ready CD20xCD3 bispecific antibody for hematological cancers, but remains partnered with Xencor on candidates for prostate cancer and B-cell malignancies.
Deal snapshot: AbbVie joins peers Merck & Co., Roche and Pfizer with a TL1A-targeted antibody for potential therapy in inflammatory bowel disease, paying $150m up front.
The US FDA has granted 510(k) clearance to a California medical technology company for a brain monitoring device that detects seizures in patients with brain injury or trauma.
Neither the mechanism nor indication is disclosed, but BMS is paying $80m up front for global rights to PRX019, which one analyst speculated might be an Alzheimer’s candidate.
CBER Director Peter Marks outlines a streamlined process to approval for treating different mutations of the same gene. NCATS’ Philip Brooks tells the Pink Sheet the approach avoids the need to “start from scratch for every new mutation.”
New draft guidance defines the necessary components of a platform technology designation request, including that sponsors demonstrate how it will save the FDA review time.
In this episode of the In Vivo podcast, Hakim Yadi, CEO and co-founder of UK techbio Closed Loop Medicine, discusses how the company is pairing software and predictive analytics with medications old and new to tailor drug dosing.
Virtual care leaders at Providence and Sanford Health shared successes and challenges in implementing remote monitoring and telehealth programs during a panel discussion at the recent Reuters Digital Health conference in San Diego.
CBER’s Nicole Verdun said the FDA is using the principles of the platform approach in situations that don’t qualify for the agency’s new incentive.
ADVERTISEMENT