Quality
Brazil looks to EU, UK and others for inspiration on introducing a regulatory sandbox.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
A government report requested by US lawmakers Debbie Dingell and Anna Eshoo to review the FDA’s postmarket surveillance of medical devices stresses that strengthening the system is critical to addressing adverse events linked to devices after they hit the market.
A bipartisan bill now in the US Senate seeks to change the classification of implantable hearing aid devices to allow Medicare to reimburse for the devices, potentially expanding access for many Americans who require them.
Cosmetics companies should familiarize themselves with current good manufacturing practices standards, namely ISO 22716:2007 and NSF/ANSI 455-3-2021, and begin building out their quality programs accordingly. HBW Insight guest columnists Marcha Isabelle Chaudry, attorney and founder of The Equity and Wellness Collaborative, and Rachel Raphael, partner at Morgan, Lewis & Bockius, offer preparation tips ahead of US FDA rulemaking.
Philips has filed a lawsuit against a Pennsylvania lab it hired to analyze sound abatement foam that prompted widespread recalls of its CPAP machines. Philips alleges PSN Labs grossly overestimated the risk to patients, which led Philips to initiate a larger recall than it otherwise would have.
While the National Electrical Manufacturers Association supports the Biden Administration’s plan to impose tariffs on a range of Chinese goods coming into the US, it also supports holding off on their implantation.
The US Food and Drug Administration released five warning letters and two close-outs last month, including two warning letters to Chinese makers of plastic syringes.
The Voluntary Improvement Program will increase the overall quality of the industry, members of the medical device quality community agreed during the MDIC Excellence and Quality Summit.
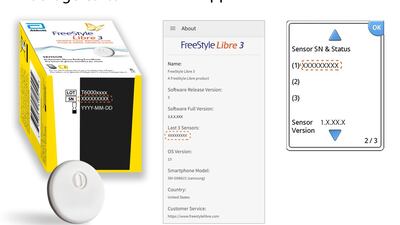
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
The US FDA has issued two new warning letters to Chinese syringe makers Jiangsu Shenli and Jiangsu Caina following inspections of their facilities. The letters are the latest in what has been an ongoing investigation into these devices.
Last month, the US FDA increased the number of injuries and deaths initially reported in a March recall from Philips, which the company disputed. The FDA now says its adjusted numbers were in error.
Manufacturers should keep a sharp eye on data from contract research organizations, looking for “any irregularities” amid a concerning trend of data integrity issues pertaining to BA/BE studies conducted by certain CROs in India, the director of the FDA’s drug center says.
Manufacturers should keep a sharp eye on data from contract research organizations, looking for “any irregularities” amid a concerning trend of data integrity issues pertaining to BA/BE studies conducted by certain CROs in India, the director of the FDA’s drug center says.
Leaders from India’s top drug makers discuss efforts to operationalize a world-class skilling institute, backed by tie-ups with organizations like the PDA, ISPE and also taking a leaf out of the automobile industry’s book to build supply chain resilience. They also exuded confidence on moving up the innovation value chain.
More than a year after the FDA created a new rule allowing hearing aids to be sold over the counter and directly to consumers, a government report finds the category has not yet made the impact many were expecting.
The US FDA has published draft guidance for developers of drug delivery devices listing recommendations related to device design outputs essential for establishing and assessing the performance of their products.
The US FDA is reporting dozens more additional deaths associated with a May recall of Philips ventilators than initially reported. The company says it stands by its original number of seven and has reached out to the FDA.
India’s drugs regulator sets out plans for extensive digitization across the regulatory value chain and regulatory rationalization initiatives, while also “looking inward” to up its game as it tightens processes and enforcement. Audit action under the revised GMP norms is also being kicked off.
Globally harmonized guidance on evaluating the viral safety of biotechnology products has undergone major revisions for the first time in over two decades to address a raft of scientific advances. Manjula Aysola explores the changes and their impact for manufacturers.
ADVERTISEMENT