Risk Management
The US FDA updated labeling for Astellas’ drug for hot flashes after a postmarketing report of a single patient with signs and symptoms of liver injury taking the medicine.
An employee of Astellas in China first detained 17 months ago on suspicion of espionage has been formally indicted and now faces trial.
Drug companies may have to adjust their benefit-risk management strategies to ensure compliance with the European Medicines Agency’s newly revised guidance on how to develop and evaluate risk minimization measures.
A draft declaration to be presented to the UN General Assembly in September suggests that a “lack of regulation of over-the-counter use of antimicrobials” is one of the “drivers of antimicrobial resistance.” Industry, however, insists that misuse and over-prescription of antibiotics are the primary drivers of AMR, and is advocating for the text to be amended accordingly.
Philips has filed a lawsuit against a Pennsylvania lab it hired to analyze sound abatement foam that prompted widespread recalls of its CPAP machines. Philips alleges PSN Labs grossly overestimated the risk to patients, which led Philips to initiate a larger recall than it otherwise would have.
Marketing authorizations for OTC medicines could be rejected if their environmental risk assessments do not meet new requirements proposed within the EU pharma legislation revision. HBW Insight speaks to regulatory law experts Tine Carmeliet and Eline D'Joos to find out what you need to know about the new rules.
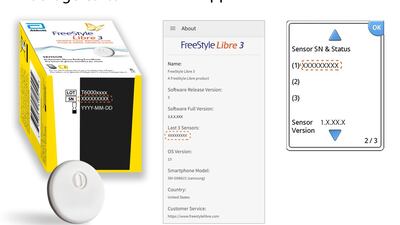
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
UK regulator the Food Standards Agency intends to assess the risks associated with consuming supplements containing ashwagandha and to determine whether a safe level of the herb can be established. The launch of the consultation follows warnings from regulators across Europe about the safety of ashwagandha, with its consumption linked to a number of adverse health effects.
The US FDA is reporting dozens more additional deaths associated with a May recall of Philips ventilators than initially reported. The company says it stands by its original number of seven and has reached out to the FDA.
The US FDA has issued its final guidance defining manufacturer behaviors it deems as hampering the agency’s ability to conduct an inspection. In a previous draft guidance, the agency expanded its longstanding policy on inspections of drug companies to include device makers as well.
The US FDA has issued its final guidance defining manufacturer behaviors it deems as hampering the agency’s ability to conduct an inspection. In a previous draft guidance, the agency expanded its longstanding policy on inspections of drug companies to include device makers as well.
EFSA-commissioned report proposes changes to the way the authority conducts consultations on its scientific assessments which could give industry more opportunities for involvement.
The European Medicines Agency is reviewing the known risk of agranulocytosis in people taking medicines containing the painkiller metamizole, which is approved for use in most of the EU member states but banned in the US, the UK and other countries around the world.
A GenAI copilot for biopharma regulatory requirements could yield significant returns.
Teleflex is recalling thousands of intra-aortic balloon catheter kits that are used during cardiac procedures and to treat complications from heart failure. This is the latest in a series of recalls from Teleflex and its subsidiary Arrow International.
Real-world data and evidence, for example generated by digital consumer health technology like apps and wearables, could provide crucial support for Rx-to-OTC switch applications, argues Sanofi Consumer Healthcare’s Penny Glover at the Association of the European Self-Care Industry's 60th Annual Meeting in Brussels, Belgium. Recent research by Sanofi indicates that regulators are actually already using RWD and RWE in many cases when assessing switch applications, even if they do not call it that.
The US FDA has issued a final rule allowing the agency to destroy some medical devices that have been refused entry into the US. The rule takes effect 1 July.
The US FDA has issued a final rule allowing the agency to destroy some medical devices that have been refused entry into the US. The rule takes effect 1 July.
In this episode of HBW Insight's Over the Counter podcast, we unpack the Corporate Sustainability Reporting Directive (CSRD) with the help of ESG expert Jasper Crone, drilling down into exactly what consumer health companies need to know about this enormously complex and significant piece of EU legislation.
Virtual care leaders at Providence and Sanford Health shared successes and challenges in implementing remote monitoring and telehealth programs during a panel discussion at the recent Reuters Digital Health conference in San Diego.
ADVERTISEMENT