Rwanda
Novartis leads the fight against malaria, pioneering innovative studies in sub-Saharan Africa to combat parasite resistance to current antimalarials.
The Pink Sheet highlights recent comments and insights from pharma officials and executives on key issues we are covering.
The Swiss major’s global health chief Lutz Hegemann tells Scrip on a trip to Rwanda that being a profitable enterprise while helping to improve access to new and older therapies for those who need it most is the real measure of a successful business.
As Congress pressures FDA to increase foreign inspections, the agency unveils plans to strengthen its overseas presence, including new offices and more staff in the New Delhi, India, post. Deputy Commissioner Kimberlee Trzeciak notes the moves are resource dependent.
Rwanda’s health minister says the new body will play a key role in promoting cooperation and mutual recognition of regulatory decisions by individual agencies across the continent.
Africa is taking steps to massively increase its vaccine production capacity and reduce its heavy dependence on other countries for supplies. Muthoni Wanyeki of the Open Society Foundations explained to the Pink Sheet how COVID-19 has highlighted the need for action and why it is important to keep up the momentum achieved so far.
The small central African country has been chosen to host the planned African Medicines Agency and is also set to be a key player in medicines and vaccines manufacturing technology on the continent.
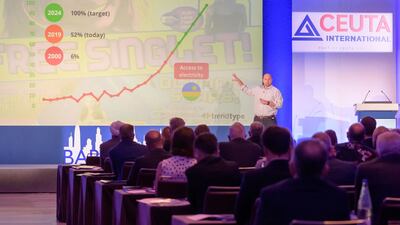
Africa represents a new frontier for health and beauty firms. With significant economic growth and a population explosion forecast for the continent, now is the perfect time to start thinking about the Africa opportunity, market expert Ben Longman told the recent Ceuta International Conference.
Catalyst Principal continues east Africa health care investing; GOED MOU with US-China Health Products; class action against IntenseX dismissed; Prime Nutrition brand under Hi-Tech roof; and more news in brief.
GlaxoSmithKline says it will no longer be filing for patent protection for its medicines in leastdeveloped and low-income countries. The firm is also set to be the first company ever to commit future oncology products to patent pooling.
Use of Merck's Gardasil and GlaxoSmithKline's Cervarix has cut infection rates in half from the human papillomavirus (HPV) in US teenage females 14-19 years since routine vaccination with the products began in 2006, researchers from the US Centers for Disease Control and Prevention (CDC) reported on 19 June, with the head of the agency calling that outcome "striking."
The GAVI Alliance has announced it is to fund vaccines against the human papillomavirus (HPV) and negotiations with the companies that market vaccines, GlaxoSmithKline and Merck & Co, are underway, said a GAVI spokesperson. The public-private vaccination initiative says it must secure a "sustainable price" from manufacturers. It is unclear what this price might be, but will likely be below $5 per dose.
Merck & Co's HPV vaccine Gardasil and Qiagen's HPV DNA tests are at the centre of Africa's first integrated cervical cancer prevention campaign that incorporates both vaccination and screening on a national level, launched this week by the Rwandan government. The programme, which Rwanda described as a world first, will show that new technology can be successfully introduced to developing countries and then other countries will likely follow suit, Rwanda's permanent secretary for the health ministry, Dr Agnes Binagwaho, told Scrip.
A private member's bill, which aims to reform Canada's troubled law allowing developing countries to import low-cost generic medicines, has been voted in by the House of Commons.
Seven Médecins Sans Frontières (MSF) measles vaccination points have been attacked by the Congolese national army in the Democratic Republic of Congo (DRC), MSF has revealed.
Members of the World Trade Organization have decided to extend to 2011 the deadline for countries to accept a 2005 amendment to the TRIPS agreement, which would allow poor countries without manufacturing capacity to import cheap generic versions of patented medicines under compulsory licensing.
After years of criticism, Canada's Access to Medicines Regime (CAMR) may finally be improved, after public hearings for a bill designed to streamline the controversial system took place last week.
Gambia is using Wyeth's older heptavalent vaccine, Prevenar, for the introduction of its national pneumococcal immunisation programme, which was rolled out in Banjul this week. The move was made possible by a Wyeth donation of 3.1 million doses of the vaccine through vaccine-funder the Gavi Alliance; some of the vaccine was used in campaigns in Rwanda in April.
A $1.5 billion scheme designed to accelerate access to vaccines against pneumococcal disease in developing countries has officially been launched. Up to 60 of the world's poorest countries are expected to benefit from the scheme with the introduction of the childhood vaccine as early as 2010.
Support to improve Canada's Access to Medicines Regime (CAMR) is gaining momentum, with the tabling of a new Private Member's Bill.
ADVERTISEMENT