Safety
Long-term follow-up requirements have taken a conservative approach but could be ripe for re-examination and global harmonization given the years of experience with the products, Kite Pharma executive director says; former FDA gene/cell therapy office head Wilson Bryan calls for elimination of the classwide REMS.
The US FDA updated labeling for Astellas’ drug for hot flashes after a postmarketing report of a single patient with signs and symptoms of liver injury taking the medicine.
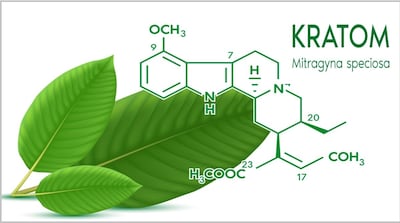
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Principal deputy commissioner Bumpus agrees with advisory committee findings that safety issues with ITCA 650 preclude approval, regardless of comparisons to other diabetes treatments. Final order caps a nearly eight-year regulatory saga that has grown increasingly rancorous.
Height of plaintiff attorney’s argument to present evidence which would prompt speculation by a jury was request to parse research by Kenvue’s lead expert, who coordinated the International Consensus Statement on ADHD by the World Federation of ADHD, where he’s president.
Database review contradicts earlier findings with Novo Nordisk’s blockbuster incretin, but its small size and flawed design mean its commercial implications will be limited.
It is “absolutely fundamental” that manufacturers of cell and gene therapies interact with inspectors from regulatory agencies to understand their expectations around good manufacturing practice, a compliance consultant at CDMO eXmoor pharma says.
A government report requested by US lawmakers Debbie Dingell and Anna Eshoo to review the FDA’s postmarket surveillance of medical devices stresses that strengthening the system is critical to addressing adverse events linked to devices after they hit the market.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to CHPA’s Mike Bailey, senior vice president of regulatory and scientific affairs; and regulatory and scientific affairs VPs Marcia Howard and Jay Sirois.
This week, surgical robot maker Globus Medical got a warning letter from the US Food and Drug Administration; the FDA cleared a hemostatic gel to stop blood loss; Medicare issued a payment code for Medtronic’s renal denervation device; and more.
Drug companies may have to adjust their benefit-risk management strategies to ensure compliance with the European Medicines Agency’s newly revised guidance on how to develop and evaluate risk minimization measures.
Amulet announces $5.8 million in series A financing led by HealthX Ventures along with Incite Ventures, AllerFund, Mendota Venture Capital and Great Oaks Venture Capital. Allergy Amulet is for on-the-go testing of food to determine whether allergens are present.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
With monographs@FDA portal down, agency moves to NextGen and extends comment period through 27 September for first OTC monograph change it proposed using its overhauled program other than 32 monographs finalizations required in 2020 legislation authorizing overhaul.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”
Germany's Expert Committee on Prescription recommends the Rx-to-OTC switch of an azelastine and fluticasone propionate combination nasal spray, the second time around. Low-cost smoking cessation drug cytisine, on the other hand, is denied - also the second time it has appeared before the committee.
Philips has filed a lawsuit against a Pennsylvania lab it hired to analyze sound abatement foam that prompted widespread recalls of its CPAP machines. Philips alleges PSN Labs grossly overestimated the risk to patients, which led Philips to initiate a larger recall than it otherwise would have.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
ADVERTISEMENT