Sustainability
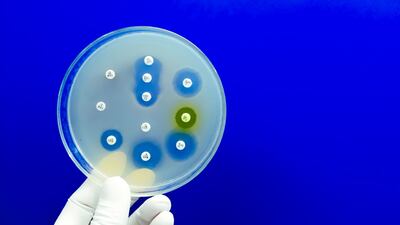
The UK will require all antibiotic manufacturers looking to apply for funding under its subscription payment model to demonstrate they are meeting waste discharge standards in a bid to reduce antimicrobial resistance.
Plastics – specifically the reduction of virgin plastic in packaging and the move to recyclable or refillable packaging – is one of nine priority topics Kenvue is focused on as part of its global sustainability strategy, according to the company’s global head of packaging innovation, sustainability and experience, David Lickstein.
Meron Mathias is vice president of CSR and sustainability at Thermo Fisher, whose specialty diagnostics division makes it the fifth leading player by revenues in the global IVD industry. In this podcast, she reflects on Thermo Fisher’s early commitment to ESG and sustainability reporting, its Scope 1, 2 and 3 targets and how regulation is moving industry towards a mandatory disclosure landscape.
The Fragrance Creators Association president and CEO discusses the group’s vision for fragrance industry stewardship and opportunities provided by AI, biotechnology and wellness trends in an exclusive Q&A with HBW Insight.
A draft declaration to be presented to the UN General Assembly in September suggests that a “lack of regulation of over-the-counter use of antimicrobials” is one of the “drivers of antimicrobial resistance.” Industry, however, insists that misuse and over-prescription of antibiotics are the primary drivers of AMR, and is advocating for the text to be amended accordingly.
Perfect Corp. identifies waste reduction at beauty retail as the most effective AI-driven strategy for improved sustainability, based on a survey of C-level industry executives summarized in its report, "The Most Influential AI Trends in Beauty and Fashion."
The E in ESG: SUDs, reuse, disposables, refurbishing, reprocessed devices? Multiple panelists at the 2024 MedTech Forum addressed the role medtech manufacturers should have in the climate change challenge.
In her role as Boehringer's senior vice president and global head of Development Sciences, Xiaorong He emphasizes patient-centric innovation and sustainability. She is committed to incorporating patient insights into product development and advancing eco-friendly practices within the company's R&D pipeline.
Marketing authorizations for OTC medicines could be rejected if their environmental risk assessments do not meet new requirements proposed within the EU pharma legislation revision. HBW Insight speaks to regulatory law experts Tine Carmeliet and Eline D'Joos to find out what you need to know about the new rules.
Consumers increasingly demand more from health and wellness brands, particularly valuing those that connect wellness with the home environment, PA Consulting's Rhea Patten explains, in this episode of HBW Insight’s Over the Counter podcast. Consumer health companies also now have a responsibility to contribute to global wellbeing, which can in turn enhance brand impact and drive business growth.
The E in ESG: SUDs, reuse, disposables, refurbishing, reprocessed devices? Multiple panelists at the 2024 MedTech Forum addressed the role medtech manufacturers should have in the climate change challenge.
In addition to sustainability, Myers noted DEI and advancing alternatives to animal testing among key priorities for the group. “We have member companies that really lead the way on many of those. We want to be able to amplify their good work and their messaging, because they are good corporate citizens” and “it’s what consumers are demanding anyway,” he said in an interview.
In this final article in a series on the ongoing amendments to the pharmaceutical reform package, the Pink Sheet looks at the tougher environmental risk assessment requirements that pharma companies are likely to face in the not-too-distant future.
Legislator education is key to checking state bills that overreach, says Ross, executive VP of government affairs at the Personal Care Products Council, discussing the rising tide of US state bills aimed at restricting ingredient use.
In this episode, HBW chats with Michael Washburn, principal at Washburn Consulting, on how producers of single-use packaging subject to extended producer responsibility laws rolling out in Oregon, Colorado and California next year can tackle the tedious work of collecting data on packaging materials.
Stefan Woxström, senior vice president of AstraZeneca, Europe and Canada, discusses the growing challenges in the industry and addresses the role big pharma must adopt in resolving them.
The European Commission’s Scientific Committee on Health, Environmental and Emerging Risks makes minor changes to its phthalates benefit-risk guidelines based on stakeholder feedback. The guidelines could be increasingly relevant given their applicability to other carcinogenic, mutagenic or reprotoxic (CMR) and endocrine-disrupting (ED) substances, lists that are bound to go on growing as the EU implements its Chemicals Strategy for Sustainability under the Green Deal.
The plastic-neutral retailer of personal care, health and wellness, household cleaning and other sustainable products has been focused on enhancing its customer experience and expanding its third-party assortment as it progresses toward long-term, profitable growth. The company revealed on 1 July that it will not be Plastic-Free by 2025, but is on track to avoid 15 million pounds of plastic by 2030.
The European Chemicals Agency’s committees for Risk Assessment (RAC) and Socio-Economic Analysis (SEAC) are evaluating sector by sector the proposed REACH restriction on PFAS and more than 5,600 public comments, reaching provisional conclusions on cosmetics at their June meetings. A date has not been set to consider PFAS uses in medical devices, which are numerous, essential, and in many cases devoid of alternatives, industry says.
The European Chemicals Agency’s committees for Risk Assessment (RAC) and Socio-Economic Analysis (SEAC) are evaluating sector by sector the proposed REACH restriction on PFAS and more than 5,600 public comments, reaching provisional conclusions on cosmetics at their June meetings. A date has not been set to consider PFAS uses in medical devices, which are numerous, essential, and in many cases devoid of alternatives, industry says.
ADVERTISEMENT