US States
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
Multi-cancer diagnostics can help get oncology patients the treatment they need more quickly, but lack of reimbursement has kept such tests out of reach for many patients. Bills providing coverage have passed or are under consideration in more than half of the states and have been introduced in both houses of US Congress.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
WishGarden Herbs marks 45 years with line extensions; Obesity doctor develops SoWell supplements for GLP-1 patients; Vital Proteins makes sustainability vital in packaging; and interior decoration and rent awards in vitaminwater campaign.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
Putative class-action complaint alleging false advertising for Tylenol Rapid Release gelcaps recently dismissed on J&J Consumer’s motion arguing that federal preemption prohibits requiring different or additional labeling for drugs available under FDA OTC monograph.
Avista VC acquires OTC CMO Trillium; FDA deputy commissioner Jones to open CRN conference; AG1 founder steps down, COO moves up to CEO; ChromaDex hires general counsel; Ajinomoto, Shiru partner to develop sweet proteins; and change at ingredient provider LBB helm.
The firm’s development of VagiBiom prebiotic and probiotic supplements and suppositories and Vee feminine health tracking app emanated from founder Bobban Subhadra’s interest in the health potential of the human microbiome and research in the fields of microbiology and immunology.
Church & Dwight’s slower sales at retail in June-July than in January-May point to still slower results for rest of year. It doesn’t expect second-half help from gummy vitamin lines or give them a full vote of confidence for remaining in portfolio.
On 29 July, Guardant Health Inc. announced it received US FDA approval for its blood test Shield to be used as a primary screening option for colorectal cancer, bringing it one step closer to gaining Medicare coverage.
P&G's FY2024 Q4 net sales were flat at $20.5bn, lower than consensus estimates, but the firm says its underlying business divisions are healthy and forecasts FY2025 sales up 2% to 4%, up 3% to 5% organically.
CRN appreciates Durbin’s “commitment to greater transparency in the dietary supplement marketplace,” but “cannot endorse it in its current form”; AHPA asks for better option; and NPA remains opposed.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Consumers increasingly demand more from health and wellness brands, particularly valuing those that connect wellness with the home environment, PA Consulting's Rhea Patten explains, in this episode of HBW Insight’s Over the Counter podcast. Consumer health companies also now have a responsibility to contribute to global wellbeing, which can in turn enhance brand impact and drive business growth.
Registrar Corp. introduces Adverse Event Management Software that ‘securely’ intakes sensitive consumer medical data, tracks adverse events for all products globally, transmits information to internal stakeholders and formats serious events to the FDA Medwatch format for submission.
Martha Stewart sells supplements on iHerb; Cheribundi partners spread the brand at Paris Games; GoodSport’s latest partner is a Bear; Herbalife-sponsored runner competes in ultramarathons; Dutch researcher joins Unicity science board; and Beacon Wellness adds sales strategy chief.
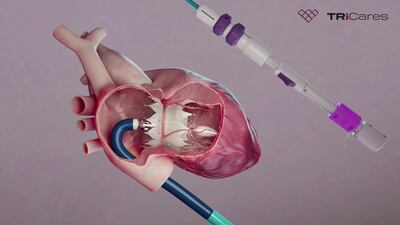
TRiCares SAS announced it raised $50m in series D funding from a single unnamed investor. The funding will support the company’s upcoming US early feasibility study and EU CE mark clinical investigation for its transfemoral tricuspid heart valve replacement system, Topaz.
In addition to sustainability, Myers noted DEI and advancing alternatives to animal testing among key priorities for the group. “We have member companies that really lead the way on many of those. We want to be able to amplify their good work and their messaging, because they are good corporate citizens” and “it’s what consumers are demanding anyway,” he said in an interview.
ADVERTISEMENT