Vaccines
Revised recommendation is based on most recent data on circulating strains in US, where KP.3 is now dominant. Novavax sticks with plans targeting the JN.1 ‘parent strain.’
Bangladesh’s Beximco reported double-digit growth both domestically and abroad in its financial first half, while continuing to expand in other international markets. Given its new launches and a COVID-19 vaccine distribution deal, the company remains optimistic about its future performance.
The company will ramp up manufacturing of its Jynneos vaccine to meet demand in worst-affected African countries and stockpiling richer nations.
2030 sales forecasts for Novo’s obesity hope are an order of magnitude larger than its closest rival among 2025’s expected debutantes.
The specialist pharma company hopes demand for mpox vaccines will help establish its vaccine platform, but cash and investor confidence remain in short supply.
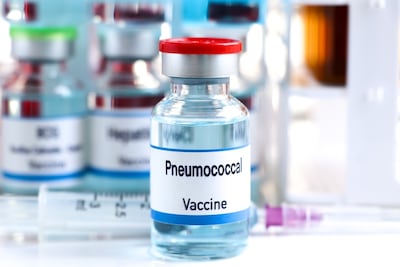
The company’s VAX-31 against 31 pneumococcal serotypes outperformed Pfizer’s market-leading Prevnar 20 and could provide the broadest coverage of any vaccine in its class.
US FDA’s 2015 accelerated approval called for confirmatory trial completion by 2018, but study initiation was delayed. Completed in 2022, the trial not only verified Bexsero’s clinical benefit, it also served as the pivotal study for GSK’s pentavalent meningococcal vaccine candidate.
To help sponsors developing candidate mpox vaccines, the World Health Organization will soon be finalizing guidance on the preferred and minimum characteristics these products must satisfy regarding efficacy, dose regimen and other aspects to secure regulatory approval.
Pfizer/BioNTech and Moderna rapidly adapted Comirnaty and Spikevax for the 2024-2025 season to address the KP.2 variant after the US FDA had advised them to target JN.1 in June.
Serum Institute is working on an mpox vaccine while other Indian firms are weighing options after the WHO sounded an alert and a new case was reported in neighboring Pakistan. Scrip looks at factors that could favor development and/or manufacture of an mpox vaccine at Indian majors.
The WHO’s emergency committee has warned of a lack of understanding of the epidemiology of mpox, the limited availability of vaccines, and the complexities of donations and procurement.
The companies said their combination mRNA vaccine showed protection against SARS-CoV-2 and influenza A, but not influenza B.
The vaccines alliance says its First Response Fund could be used to cover the cost of doses and has outlined plans for a vaccine stockpile. Some experts have criticized the slow rate of response to mpox and called for more solidarity on vaccine provision at global level.
The European Medicines Agency has started reviewing new marketing applications for 10 products, including Blenrep, GSK’s previously approved multiple myeloma drug that was withdrawn from the market, and vimseltinib, which could become the EU’s first oral treatment approved for TGCT.
The Africa CDC says the donation of more than 215,000 doses of mpox vaccine is a “crucial step” in tackling the latest outbreak.
An emergency use listing means the vaccines can be approved for use in lower-income countries where they are not licensed, and will allow international organizations like Gavi and UNICEF to procure them for wider distribution.
The drug maker previously said approval in adults 18-59 could drive some evolution in a space where 60 mostly remains the lower limit for recommended use of RSV vaccines.
One company’s weak quarterly sales should not make a pharmaceutical winter. But combine the lower sales of major vaccine families across three companies’ portfolios with weak demand in China and it starts to feel chilly.
HPV vaccine shipments to China are expected to fall after a government crackdown on bribery and corruption caused scientific communication with health care providers to decline. Gardasil also may be hurt by budgetary pressures on distribution of the government-reimbursed vaccine.
Second quarter revenues are up but RSV jab woes drag on the company’s stock.
ADVERTISEMENT