Warning Letters
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
This week, surgical robot maker Globus Medical got a warning letter from the US Food and Drug Administration; the FDA cleared a hemostatic gel to stop blood loss; Medicare issued a payment code for Medtronic’s renal denervation device; and more.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
Consumer health, particularly food supplements that make unauthorized medical or health claims, has been a key area of focus for the UK Advertising Standards Authority's recently introduced Active Ad Monitoring system, which captures ads by relevant advertisers from a range of social media platforms, and applies machine learning algorithms to identify and flag likely non-compliant ads, which are then sent to experts to review and act on. This infographic highlights recent ASA rulings against ads for supplements claiming to treat anxiety and stress, menopause, autism/ADHD and weight loss, which were flagged by AI.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
The US Food and Drug Administration released five warning letters and two close-outs last month, including two warning letters to Chinese makers of plastic syringes.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
The US FDA has issued two new warning letters to Chinese syringe makers Jiangsu Shenli and Jiangsu Caina following inspections of their facilities. The letters are the latest in what has been an ongoing investigation into these devices.
This week, Nipro Medical Corp. announced it will invest $397.8m to build a US-based production plant, generating 232 new jobs; both Baxter and Hamilton announced ventilator recalls; Imperative Care wins FDA clearance for its stroke catheter; Intelligent Ultrasound Group plc entered into a conditional sale and purchase deal to sell its Clinical AI business to GE HealthCare for £40.5m; RMI distributed 350m rapid test kits in the fight against HIV/AIDS; Jiangsu Shenli Medical Production Co., Ltd received a second FDA warning letter about quality and safety of plastic syringes.
Noting similar warnings a year ago, the FDA and FTC announce warnings to six more, part of a joint effort to stop sales of copycat food products containing delta-8 THC, saying companies selling these "illegal products are demonstrating complete neglect for consumer safety.”
Analysts suggest more and larger investments than president’s recent $1m and $5m private placements needed to last long enough to establish sales of its functional beverage sufficient to turn a profit. Results expected soon from clinical trials on “detoxifying the alcohol out of” users’ bodies.
The US Food and Drug Administration released two warning letters and two close-outs last month, with one close-out resolving a finding the Owlet Smart Sock was being sold without FDA approval.
The US Food and Drug Administration released five warning letters last month, showing an ongoing focus on premarket clearance and the quality system regulations compliance.
The US Food and Drug Administration released eight warning letters and one close-out last month. Big names caught in the net included Beckman Coulter, Royal Philips and Cardinal Health.
Without additional funding, FDA’s ORA faces challenges in retaining and hiring staff, which will impact inspections, says office chief Michael Rogers.
This week, Philips Respironics reached a $1.1b settlement affecting CPAP and other breathing devices. Toku announced it received US FDA breakthrough device designation for its MyKidneyAI technology. This May, the FDA will hold its REdI conference focusing on innovation in medical product development and hold another townhall focusing on considerations for selecting a sterilization modality.
The number of class I medical device recalls has increased over the last decade, and that concerns officials at the FDA.
The US Food and Drug Administration released seven warning letters in March 2024, of which three were related to a recent crackdown on China-made syringes. Other letters recounted troubles at Exactech, Fresenius and ReNovo, as well as clinical trial violations from Nobles Medical Technology.
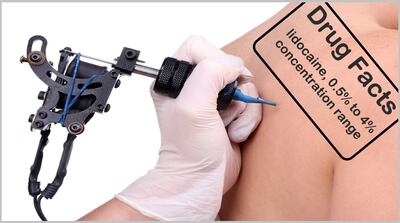
FDA warns six businesses found selling directly to consumers topical formulations containing lidocaine outside the 0.5% to 4% concentration range allowed under OTC monographs for topical analgesics
Agency wants more information about API suppliers as it winds up case against KV Tech for hidden use of Dr. Reddy’s plant and seeks to find disappearing manufacturer of contaminated OTC eye drops.
ADVERTISEMENT