Wound Healing & Tissue Repair
Cresilon CEO Joe Landolina says the newly FDA-cleared Traumagel for moderate and severe bleeding is easier to use than many currently available solutions like gauzes and sponges, and provides faster results.
After a quiet July, August and September are shaping up to be busy on the approvals front. Already this month, the US FDA has approved Adaptimmune’s Teclera for synovial sarcoma, Phathom’s Voquezna for gastro-esophageal reflux disease and Servier’s Voranigo for gliomas. Here, Scrip takes a look at ten other approvals for novel products in the offing for the third quarter.
SanBio’s lead cell therapy asset has been on a bumpy journey to its global-first approval and while a nod has now come in Japan in a high-need indication, a commercial launch is conditional on additional data to establish product equivalence and manufacturing consistency.
Breathomics, UTI diagnosis and advanced wound healing innovations were among the center stage technologies at BioWales in London 2024. Hear from CEO John McKinley on Imspex Diagnostics' plans in embedded podcast.
Breathomics, UTI diagnosis and advanced wound healing innovations were among the center stage technologies at BioWales in London 2024.
A novel robotic-assisted therapy could improve the lives of men with benign prostatic hyperplasia, real-world data presented at the annual meeting of the American Urological Association suggests.
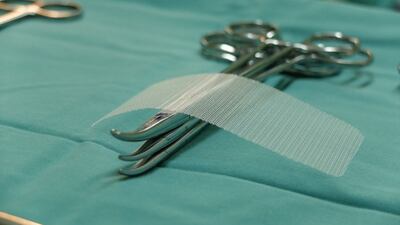
The US FDA continues to provide updates on the safety and efficacy of surgical mesh sling procedures, a common surgery to correct stress urinary incontinence.
Tokyo-based Healios will lead the global clinical studies for Athersys’ cell therapy MultiStem for ARDS and start US Phase II trials for trauma following their long-term collaboration.
While the latest missions from NASA may seem like the stuff of science fiction, discoveries from outer space are not only unlocking the mysteries of the cosmos, but improving technologies used every day on Earth, including those in the medtech industry.
The US FDA says consumers, providers, and facilities should not use recalled saline and sterile water medical products manufactured by Nurse Assist that were sold under various brand names.
The US FDA is reminding the public that there are no surgical mesh products that have been cleared or approved by the agency for use in breast surgery.
After an inspection of its Boston facility, the US FDA issued a warning letter to Integra LifeSciences' subsidiary TEI Biosciences for distributing collagen-based medical devices that failed bacterial endotoxin tests and did not conform to good manufacturing practices.
The Food and Drug Administration is providing patients with information on the pros and cons of hernia mesh surgery, a procedure that has come under intense scrutiny in recent years.
Coloplast expects Kerecis' rapid growth to justify the high price of the acquisition. The Icelandic company's skin-substitute products are made from minimally processed cod skins.
While perhaps best known for historical achievements in immuno-oncology and CNS disorders, the Swiss biotech sector is slowly cultivating a new area of expertise in dermatology. Scrip spoke with two firms applying cell therapy technologies to the challenging area of chronic and acute wounds.
Vertex has invested heavily in advancing its stem-cell based candidates for type 1 diabetes, but Sernova has inched ahead in the race to bring a functional cure for the condition to patients.
The Digital Therapeutics Alliance (DTA) released a standardized definition and guidance for digital therapeutics during its annual meeting in Washington, DC. The move comes as part of a push toward standardization for the fast-changing field.
After consulting with the US FDA, Integra Life Sciences initiated a recall of products produced in its Boston Facility. The company expects losses of $22m.
The company estimates there are about 9,000 reimbursable patients with dystrophic epidermolysis bullosa worldwide and anticipates a third-quarter launch following FDA approval.
The Swedish firm’s lead program has unexpectedly disappointed in a Phase II scar prevention trial, prompting it to turn its efforts to its only other clinical asset for the treatment of chronic wounds.
ADVERTISEMENT