Wound Management
Amferia is developing an antimicrobial peptide hydrogel dressing to combat infections in acute wounds in humans and veterinary contexts. Supported by €1.2m of new venture capital financing, the company is planning pivotal clinical studies to support an FDA application later this year and CE mark submission in 2026-2027.
Cresilon CEO Joe Landolina says the newly FDA-cleared Traumagel for moderate and severe bleeding is easier to use than many currently available solutions like gauzes and sponges, and provides faster results.
Breathomics, UTI diagnosis and advanced wound healing innovations were among the center stage technologies at BioWales in London 2024. Hear from CEO John McKinley on Imspex Diagnostics' plans in embedded podcast.
Breathomics, UTI diagnosis and advanced wound healing innovations were among the center stage technologies at BioWales in London 2024.
Medtech Insight's News We're Watching highlights medtech industry developments that you may have missed over the last few weeks. Chinese researchers are working on a self-powered pacemaker, Carmat updated its artificial heart progress, a trial validates Smith & Nephew's Regeneten implant in shoulder surgery, experts back intravascular IVUS in peripheral interventions, and Spectral AI launches a new trial of its AI for burn evaluation.
Integra will pay $275m in cash plus $5m more in potential milestone incentive payments to add Acclarent’s ear, nose, and throat surgery devices to its existing neurosurgery portfolio.
This week, the Biden administration announced a new council on supply chain resilience that includes health care goals; an apparent enforcement surge against device fraud continued; Siemens won a $5.5m grant to develop a better sepsis test; and the FDA proposed new classifications for wound care products.
The diagnostic company has secured more government funding for DeepView, a product it has developed to assess the healing of certain types of wound. In total, BARDA has supported SpectralMD with almost a quarter of a billion dollars.
The Swedish company has developed a proprietary method of personalizing venous grafts, and plans to bring it – alongside some other products – to the market.
The FDA issued warning letters to three US device firms last month, as well as closing out a 2021 letter to Invacare.
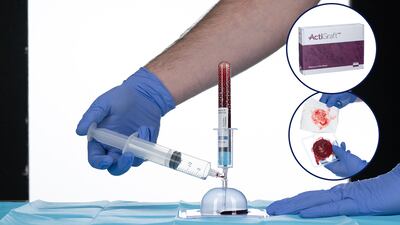
The ActiGraft PRO treatment for diabetic ulcers recently procured Medicare coverage nationwide, despite anti-abuse policies that have made reimbursement for new wound care products rare. Company general manager Robert Mueller talked about the process.
After an inspection of its Boston facility, the US FDA issued a warning letter to Integra LifeSciences' subsidiary TEI Biosciences for distributing collagen-based medical devices that failed bacterial endotoxin tests and did not conform to good manufacturing practices.
Coloplast expects Kerecis' rapid growth to justify the high price of the acquisition. The Icelandic company's skin-substitute products are made from minimally processed cod skins.
While perhaps best known for historical achievements in immuno-oncology and CNS disorders, the Swiss biotech sector is slowly cultivating a new area of expertise in dermatology. Scrip spoke with two firms applying cell therapy technologies to the challenging area of chronic and acute wounds.
After consulting with the US FDA, Integra Life Sciences initiated a recall of products produced in its Boston Facility. The company expects losses of $22m.
The wound healing giant has purchased technology from the UK-based company 30 Technology, creating speculation regarding when and how it will incorporate it into its own products.
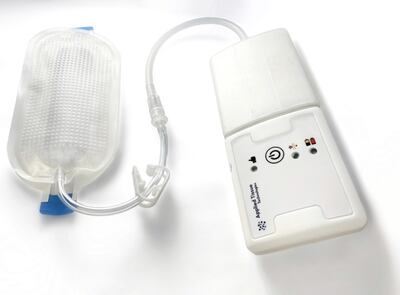
The FDA granted 510(k) clearance to Applied Tissue Technologies for its non-foam negative pressure wound therapy system.
The company’s health businesses include product lines worth nearly $9bn in annual sales. The deal is designed to allow both the new health care company and the “new 3M” to focus resources more efficiently on their separate priorities.
The Dallas-based company is using its DeepView technology to aid the treatment of hard-to-heal and chronic wounds.
Deepak Nath will take over as Smith & Nephew’s new CEO on 1 April, joining the orthopedics and wound care company after four years as president of Siemens Healthineers’ diagnostics business. Smith & Nephew’s current CEO Roland Diggelmann is stepping down by mutual agreement.
ADVERTISEMENT