Surgery
In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.
This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
Tim Schmid, executive VP and worldwide chairman of J&J MedTech, expects Shockwave, acquired in April, and Abiomed, purchased in late 2022, to be “long-term gems” for the company. Cardiovascular is among higher-growth segments where J&J has concentrated investments in recent years, along with robotic surgical systems.
This week, a Delaware court awarded Auris Health shareholders $1bn in a lawsuit against Johnson & Johnson; Abbott recalled some FreeStyle Libre 3 sensors; and McKesson purchased a controlling interest in a Florida cancer care chain.
Inari Medical is updating use instructions for a clot-removing catheter due to the potential for serious adverse effects, including death.
The Acton, MA-based tubeless insulin pump specialist expands its indication for the Omnipod 5 AID system beyond type 1 diabetes as FDA authorization for type 2 comes sooner than Wall Street expected. Analysts expect clearances of rival systems from Tandem Diabetes Care and Medtronic in 2025, but believe Insulet is well-positioned to compete.
This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.
Getinge says it plans to pay about $477m for organ transport and services company Paragonix Technologies in an effort to “redefine the market standard in transplantation.”
J&J buys heart failure implant company V-Wave, whose Ventura Interatrial Shunt could be the first device of its kind aimed to reach the roughly 800,000 patients in the US who experience heart failure and reduce ejection fraction every year.
Announced in conjunction with a $97m Series D financing round, Neptune underscores its gastrointestinal focus and robotics aspirations by spinning out Jupiter Endovascular, which will leverage $21m of the funding to support ongoing development of its Endoportal Control technology.
Caresyntax said it will use the $180m it recently raised in a series C extension and debt financing round to build out its vendor-neutral surgery platform aimed to help surgeons with real-time and long-term decision support to improve patient outcomes and efficiencies.
Medtronic receives US FDA approval for deep brain stimulation surgery for people with Parkinson’s and essential tremors while the patient is under general anesthesia, making it the first and only company to offer DBS surgery while the patient is asleep.
This week, Medtronic recalled a nerve monitoring system due to reports of false responses. The US FDA approved the first auto-injector for opioid-overdose, made by Purdue Pharma. The agency granted de novo authorization for Labcorp’s PGDx elio plasma focus Dx used by labs for genetic profiling. As of 7 August, 950 AI/ML devices have been approved by the FDA. EKO Health teamed up with LSU to help detect arrhythmias and murmurs in student-athletes.
J&J Medtech announced the FDA clearance of its dual-use robotics platform Velys Spine with plans to go to market in the first half of 2025.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
CMR Surgical has launched a multicenter pediatric clinical trial for its Versius surgical robot system across three UK sites. The trial will enroll 150 children for urological procedures, with patient outcomes followed for up to a year post-surgery.
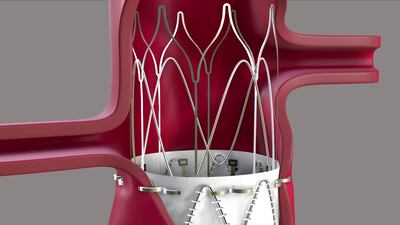
This week, Edwards announced that it has purchased JenaValve and Endotronix; a New Jersey lab has agreed to pay the government $5m for violating anti-kickback law; eCential Robotics’ spine platform made its debut for human use; and more.
In this month’s Digital Health Roundup, Medtech Insight’s Marion Webb highlights AI discussions at the HLTH Europe conference and an interview with Motif Neurotech's CEO Jacob Robinson. Elizabeth Orr discusses DeepWell DTx's newly launched VR game for treating stress-related hypertension and anxiety. Natasha Barrow provides an overview of Digital Mental Health Technologies regulation in the UK and Brian Bossetta reports ‘the good and bad’ from Medtronic's report on digital technologies' use in the operating room.
Quantum Surgical is on a mission to democratize minimally invasive cancer treatment. Its surgical robot Epione can treat inoperable abdominal and lung tumors using ablation. The company has treated over 500 patients across Europe and the US and secured €30m in funding to fuel expansion into Asia and into new cancer indications.
Endoron Medical won $10m in series A funding from European venture capital firm Sofinnova Partners and the European Innovation Council Fund. The capital will propel Aortoseal – Endoron's endograft stapling solution for abdominal aortic aneurysm – through clinical validation in its US Food and Drug Administration early feasibility study.