AI
In this week’s Digital Health Roundup, Medtech Insight’s Ryan Nelson highlights Click Therapeutics’ FDA-cleared digital therapeutics (DTx) for depression and Sinaptica Therapeutics’ personalized neuromodulation for Alzheimer’s patients. Marion Webb discusses her interview with MindMaze’s John Krakauer on their gaming-focused DTx to help people recover from serious brain injuries. Elizabeth Orr introduces new voting members of the new Digital Health Advisory Committee and Natasha Barrow discusses Hello Heart’s new symptom-tracking feature in their heart-focused app.
This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
A Deep Dive into Evidence-Based Guidelines, Clinical Considerations, and Evolving Practices for Post-SBRT Imaging in Spinal Tumor Patients
This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.
While not statistically significant, the results of a new study – which linked use of Alife Health’s Stim Assist AI tool to increased egg yield and reduced FSH usage in 291 in vitro fertilization patients versus a control arm – are encouraging, investigators say.
Caresyntax said it will use the $180m it recently raised in a series C extension and debt financing round to build out its vendor-neutral surgery platform aimed to help surgeons with real-time and long-term decision support to improve patient outcomes and efficiencies.
Hello Heart has introduced a symptom tracking feature in its app, allowing users to log feelings of dizziness or shortness of breath in conjunction with blood pressure readings. The enhancement will help all users to monitor cardiovascular risks, but women in particular could benefit, the company suggests.
Avenda’s chief operating officer Brit Berry-Pusey spoke to Medtech Insight about the company’s CPT III code for its prostate cancer mapping AI and how regulatory bodies can align to support innovators.
This week, Medtronic recalled a nerve monitoring system due to reports of false responses. The US FDA approved the first auto-injector for opioid-overdose, made by Purdue Pharma. The agency granted de novo authorization for Labcorp’s PGDx elio plasma focus Dx used by labs for genetic profiling. As of 7 August, 950 AI/ML devices have been approved by the FDA. EKO Health teamed up with LSU to help detect arrhythmias and murmurs in student-athletes.
Big Health announced it received FDA clearance for its digital therapeutics for insomnia and hopes a new CMS fee schedule will pave the way to reimbursement.
Medtronic and Abbott announced partnership to integrate Abbott’s CGM sensor with Medtronic’s insulin pump, which marks a major departure from Medtronic’s former “closed-system” approach. Medtronic also announced FDA clearance of its Simplera CGM, paving the way for its use in smartpens.
In an interview with Medtech Insight, Matt Bettonville, investor at cancer-focused venture firm Yosemite, discussed its criteria for evaluating potential investments in oncology and his outlook on the future oncology landscape.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
GE HealthCare announced it will team up with AWS to build new generative AI models and applications that can help doctors find key patient information faster and help with diagnosing and treating patients.
The UK MHRA’s AI Airlock will provide a testing ground to ensure the safety and reliability of artificial intelligence-based devices used by the National Health Service.
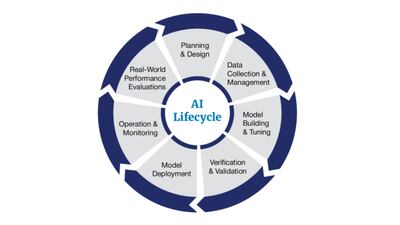
A new AI lifecycle management framework from the US FDA’s Digital Health Center of Excellence introduces considerations for the seven steps of the AI lifecycle.
Biolinq’s CEO Rich Yang spoke to Medtech Insight about the company’s wearable patch in development, which uses tiny microsensors to measure, for now, glucose, with ample runway for additional indications down the line. If approved by the US FDA, the device would become the first of its kind to monitor glucose levels in diabetes patients not using insulin.
In this month’s Digital Health Roundup, Medtech Insight’s Marion Webb highlights AI discussions at the HLTH Europe conference and an interview with Motif Neurotech's CEO Jacob Robinson. Elizabeth Orr discusses DeepWell DTx's newly launched VR game for treating stress-related hypertension and anxiety. Natasha Barrow provides an overview of Digital Mental Health Technologies regulation in the UK and Brian Bossetta reports ‘the good and bad’ from Medtronic's report on digital technologies' use in the operating room.
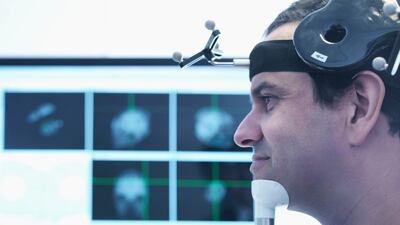
CEO Ken Mariash and scientific co-founder Giacomo Koch discuss plans for a Phase 3 trial in 2025 supporting Sinaptica’s combination of transcranial magnetic stimulation, electroencephalography and machine learning to slow Alzheimer’s progression. Potential synergies with anti-amyloid drugs, reflections on the US FDA’s TAP program, and insight into the company’s machine learning capabilities.
The final text of the AI Act is now available for all to read but, for medtech, it may feel that work is only just beginning.