United Kingdom
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.
UK-based Biocomposites will begin selling in the UK its next-generation osteoinductive bone graft substitute, NanoBone, which came with its acquisition of Artoss GmbH in June 2023. Meanwhile, the company has purchased remaining shares in the manufacturers of SYNICEM and Subiton antibiotic bone cements and preformed antibiotic-loaded spacers.
The firm’s dual-chamber leadless pacemaker system with is expected to be a growth driver for Abbott and already has acquired approximately 30%-40% of market share from Medtronic since its approval by the US FDA in July 2023, according to GlobalData.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 80 documents have been posted on the tracker since its last update.
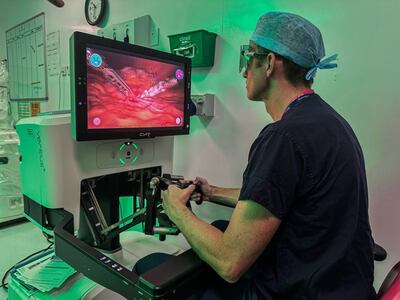
CMR Surgical has launched a multicenter pediatric clinical trial for its Versius surgical robot system across three UK sites. The trial will enroll 150 children for urological procedures, with patient outcomes followed for up to a year post-surgery.
The UK MHRA’s AI Airlock will provide a testing ground to ensure the safety and reliability of artificial intelligence-based devices used by the National Health Service.
Medway NHS Foundation Trust joined forces with Digostics to offer home oral glucose tolerance testing for pregnant women at risk of gestational diabetes.
Medical device regulatory reliance and recognition of third-party regulators’ approvals have been making news in the UK, but in certain other markets the practice is well established. International regulatory experts explained their experiences at the MedTech Forum 2024.
One week after winning the UK general election on 4 July, the Labour Party ordered a review of the National Health Service, to be published in September.
Cumulus Neuroscience has been granted £1.2m from Innovate UK to validate AccelADx, an AI-powered, non-invasive Alzheimer’s screening test that combines EEG and tablet-based assessments, as part of a transatlantic study investigating digital and blood-based biomarkers for Alzheimer's dementia.
Taylor Wessing partner Alison Dennis explains why post market surveillance is an MHRA priority as Great Britain’s medical device regulatory system takes shape.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 50 documents have been posted on the tracker since its last update.
UK medical devices manufacturer Owen Mumford moves into consumer health with umbrella brand Clariti and OTC relaunch of at-home vaginismus and dyspareunia treatment Amielle Comfort.
The UK’s national rollout of hybrid closed loop (HCL) technologies marks NHS England as the global leader in providing equitable and fair access to next-generation diabetes management, Partha Kar, type 1 diabetes and technology lead, told Medtech Insight. Market leader Medtronic offers perspective.
Implantica AG continues European rollout of RefluxStop, a non-active, laparoscopically implanted device for treating gastroesophageal reflux disease (GERD) that offers key advantages over other surgical interventions and drug therapy, according to the company. CEO Peter Forsell discusses global market opportunity and the firm’s growing body of research to support reimbursement.
Breathomics, UTI diagnosis and advanced wound healing innovations were among the center stage technologies at BioWales in London 2024. Hear from CEO John McKinley on Imspex Diagnostics' plans in embedded podcast.
The principles urge device makers to look at the whos, whys and whats of device use in developing their data transparency approaches.
Australia-based Nexsen BioTech is developing StrepSure, a rapid lateral flow test for detecting Group B Streptococcus (GBS) infection in pregnant women. Designed to provide results within 15 minutes, with the potential to save "millions of babies’ lives,” StrepSure will be assessed in a 5,000-patient clinical trial targeted for summer 2025, which will support Australia and US submissions for market authorization. Thomas Hanly, Nexsen managing director, discusses.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Sixty-five documents have been posted on the tracker since its last update.
The MedTech Forum looked at why industry should see the outcome of European Parliament elections as an opportunity to help drive through much-needed change.