AI
The Artificial Intelligence Council takes over the work that three different entities in the US FDA’s drugs center had been performing. The new, centralized entity will develop and promote consistency of AI-related activities and advance innovative uses.
Pharmaceutical companies should only use AI in evidence generation and reporting where there is “demonstrable value from doing so,” according to England’s health technology assessment body, NICE.
Shanghai issues a new set of policies and financial incentives designed to support and speed up regulatory and commercial activities in the biopharma sector, in a comprehensive stimulus package for companies based in the major Chinese city.
From the US FDA’s ISTAND Program to EU’s Melloddy Initiative, and from the global challenges of real-world data to the opportunities in India, Parexel’s EVP for Clinical Data and Digital Services, chief strategy officer and India head speak on a range of topics in this interview with the Pink Sheet.
As well as planning action to improve the EU’s competitiveness in the life science sector, the European Commission has confirmed that it will propose new legislation to reduce the bloc’s reliance on overseas suppliers of medicines as a way of tackling drug shortages.
Europe’s Innovative Health Initiative, a public-private funding partnership, has put out a call for a research project that could help analyze the use of regulatory sandboxes in health care innovation in addition to three others.
The tab 1 task is going well so far.
Pink Sheet reporter and editors discuss what the FDA included in its long-awaited guidance on clinical trial diversity action plans, along with what was left out, as well as an upcoming guidance on the use of artificial intelligence in regulatory decision-making.
The Center for Drug Evaluation and Research’s medical policy chief says artificial intelligence can aid patient recruitment and increase trial diversity, but warned of “unique” potential pitfalls for sponsors.
Draft guidance will offer a risk-based framework for accessing the credibility of AI and help ensure that AI models used to answer regulatory questions are sufficiently credible for a particular ‘context of use,’ CDER’s Tala Fakhouri says.
FDA leadership weighs in on limitations of AI as part of rollout of new technology meeting program that looks to give industry and other stakeholders a chance to inform future regulation.
AI-generated vaccines, fast-moving regulatory systems and scalable manufacturing platforms will be key in preparing for future pandemics, according to vaccine and immunology experts including the CEOs of CEPI and Gavi.
In an interview with the Pink Sheet, Troy Tazbaz, director of the US FDA's Digital Health Center of Excellence, says the agency lacks authority to regulate assurance labs, which would be used to help AI developers ensure their models are working correctly before submitting them for approval.
Insights from Day Three of the BIO International Convention in San Diego include Amgen CEO Bob Bradway taking aim at the "Innovation Reduction Act," Novo Nordisk's business development head talking about spending its semaglutide bounty, Roivant's long view on BD prospects for Immunovant's FcRn inhibitor, and more regulatory concerns around artificial intelligence.
Insights from Day Two of the BIO International Convention in San Diego include user fees potentially supporting the FDA's AI ambitions, the evolving pros and cons of partnering, and J&J's view on dealmaking in 2024.
Faster, more powerful and able to handle voices and visual images, newly released AI platform GPT-4o could potentially accelerate many tasks currently handled by pharma firms' medical affairs professionals, who are exploring ways to keep themselves relevant while embracing the unprecedented technology, DIA China hears.
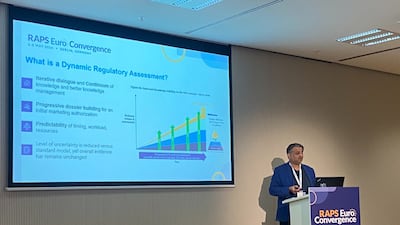
Dynamic regulatory assessments, also known as rolling or phased reviews, could see medicines granted marketing authorizations more quickly, but the EU is lagging behind the US and UK in implementing them, Gilead’s EU regulatory policy lead has said.
The ability to quickly scour the internet for comments on drugs may make it challenging to weed out true adverse events from junk as Senate Republicans consider AI-related changes to FDA's authority.
The EMA received more than 1,000 responses to its draft reflection paper on AI in drug development, some of which will be incorporated into the final document, an agency representative told this week’s RAPS Euro Convergence 2024.
A public-private partnership or other solution may be needed for the FDA to handle the costs of reviewing the growing number of drug applications with AI and machine learning components, CDER Director Patrizia Cavazzoni said.