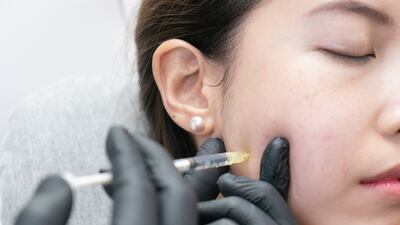
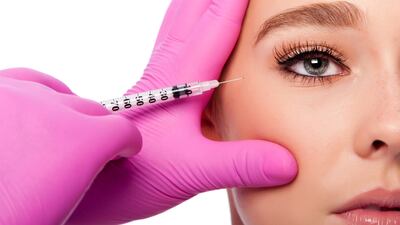
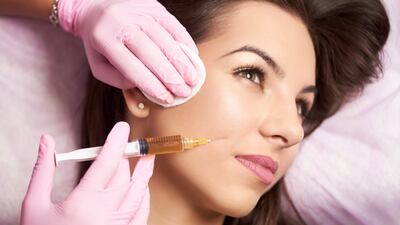
Aesthetics
The EU is putting more flesh on the bones of the requirements for Annex XVI products as two guidances are issued in quick succession.
The EU’s Medical Device Regulation regulates a small group of products with a mainly aesthetic purpose for the first time. How much notified body capacity will these potentially risky products demand?
Austrian company Croma-Pharma is the trailblazer with its newly certified dermal filler.
Similar to its post-approval strategy in aesthetics less than a year ago, Revance will initially target a small number of doctors as it Daxxify to market in the US for cervical dystonia with a broader launch in 2024.
Lawyer applauds “courageous reaction” of manufacturers of devices without an intended medical purpose which fall under Annex XVI to the Medical Device Regulation and whose products are threatened by the delays in the publication of common specifications.
The European Medicines Agency has completed its investigation into the safety of nomegestrol and chlormadinone, and has started reviewing risks linked to the cough suppressant, pholcodine, and the epilepsy and migraine drug, topiramate.
The European Commission has launched an initiative to ensure products without a medical purpose that fall under the Medical Device Regulation are appropriately classified by risk, and subject to the same pre- and post-market requirements as comparable medical devices.
Emerging Company Profile: The US biotech is developing a series of recombinant fusion proteins to tackle alopecia and other diseases without supressing the immune system.
The US agency is re-evaluating its criteria for certain human cells, tissues and cellular and tissue-based products (HCT/Ps) that are not ideally regulated as BLAs. A 2014 guidance on cord blood products could serve a template, industry suggests.
Viatris remains hopeful of obtaining FDA approval for its proposed biosimilar to Botox in partnership with Revance Therapeutics in 2026, after the latter’s manufacturing problems for another drug candidate stymied development of the rival to the world-renowned cosmetic brand.
The US agency has qualified the FACE-Q | Aesthetics patient-reported outcome instrument through its MDDT program.
The line, using colloidal oatmeal for eczema, salicylic acid for psoriasis or pyrithione zinc for flaky scalp, was independently launched in beta by a Vancouver, BC, start-up in January 2020 and debuted in May that year.
At last, common specifications for different groups of products that do not have an intended medical purpose but are covered in the scope of the EU’s Medical Device Regulation, are on the way.
There is a new legal development in the PIP breast implant scandal concerning the liability of German notified body, TÜV Rheinland. But how solid is the most recent ruling?
The US agency will hold a meeting of its plastic surgery devices panel to discuss risks associated with, and patient preference for, dermal fillers.
Paion’s novel sedation drug, remimazolam, was approved in the US, China, and Japan this year and now the European Medicines Agency is set to decide whether it should be authorized for use across the EU.
The latest drug development news and highlights from the Pink Sheet’s US FDA Performance Tracker
Public Company Edition: The former Allergan CEO is taking a special purpose acquisition corporation public to generate $460m for deals in familiar biopharma niches. Also, IPOs are expected to keep up a robust pace in the fall and Albireo raises $160m on the success of its drug for pediatric liver diseases.
The end of the traditional EU holiday period is nearing, and the pace of regulatory activity is expected to pick up sharply as September gets underway. What can medtech expect for the rest of 2020 and into the first half of 2021, in these unprecedented times?
USITC’s initial decision in the Medytox and Daewoong botulinum dispute is favorable to Medytox and poised to take a toll on the Korean firm and partner Evolus as they face a 10-year ban on imports of their product to the US.
ADVERTISEMENT