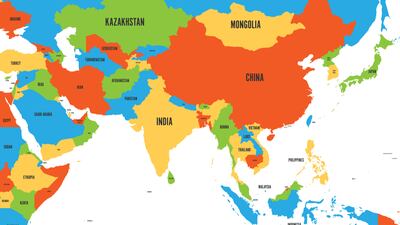
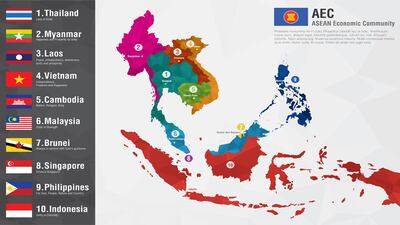
ASEAN
The Philippines medicines regulator explains how it intends to implement the ASEAN mutual recognition agreement under which member states have committed to accept bioequivalence study reports for generics issued by approved BE centers.
The 25th IMDRF meeting in March 2024 agreed that regulatory reliance models save resources, encourage innovation, bring devices to the market faster and boost patient access. Singapore and Thailand have operated a model of mutual regulatory reliance for three years. Thailand also recently broached deeper collaboration with Brazil’s Anvisa.
Digital data capture is rife now in the medtech sector. What needs to be done at an international level to control and optimize its use and that of real-world evidence based on this data? Do initiatives that came up at the International Medical Device Regulators Forum offer solutions to prevent potential global chaos?
Rising Leader Jing Lim is part of the team getting 3D implant and bone regenerating innovator Osteopore better known outside its founding base in Singapore. The company stands on the cusp of accelerated growth on the back of an expanding portfolio and its best practice approach to business building.
Governments in the ASEAN bloc continue to build national digital health roadmaps, which Siemens Healthineers’ local head of digitalization Virginia Chan sees as encouraging from many standpoints.
Vietnam’s medtech industry breathed more easily when the new decree, 98/2021, became the regulatory instrument for the sector on 1 January. While its practical approach to industry’s needs was welcomed, new price and profit declaration demands will be harder for companies to swallow.
Singapore is already deeply involved in “commercial” regulatory affairs, but it has the potential to increase the volume of “technical” RA work done locally. So says regulatory professional Jing Lim, who explained these definitions to Medtech Insight.
It has been a hard 18 months for those who rely on networking, gatherings and the personal touch to secure new business and ongoing customer loyalty. The ABHI’s Paul Benton explains how UK medtech has maintained a high profile globally in uncertain times.
The EU Medical Device Regulation will have impacts in countries beyond the EU27, a fact that is keeping MedTech Europe’s international team very busy.
More parts of the Thai FDA’s new medtech regulations will come into force in mid-March, following the first tranche last month. Other changes will become effective later in 2021 and certain elements of the system remain a work in progress.
Medtech industry representatives from ASEANMed member economies, notably Singapore, Malaysia and Indonesia, provided regulatory updates at the February Asia Regulatory Roundtable. These roundtables are organized by the ARQon consultancy and the Asia Regulatory Professional Association (ARPA). They are sponsored by Medtech Insight.
Medtech industry representatives from Thailand, Myanmar, Indonesia, Singapore and the Philippines gave updates on regulatory actions being taken to control COVID-19 and other regulatory initiatives at local and ASEANMed level during a roundtable session.
The fast-tracking of COVID-19 relevant health care products should be a blueprint for a systemic approach to matching innovation with clinical needs, say Asian medtech regulatory experts.
Siemens Healthineers' product mix of diagnostics and imaging products meant it was in the middle of a very dynamic situation when COVID-19 struck.
The annual APACMed meeting in Singapore this month will open its doors to physical medtech industry attendees in a hybrid format that could become the norm.
The Asian Medtech Associations Regulatory Networking discussions this month centered on strategies to control COVID-19 locally and the activities of the ASEANMed industry association. This editorial feature is hosted by Medtech Insight, along with the ARQon Asia Regulatory and Quality Consultancy and the Asia Regulatory Professional Association. This is the second of two articles on the discussions.
Thailand has opted to align with IMDRF principles on the classification of devices. The change also brings the Thai FDA into line with Asean Medical Device Directive principles.
The spread of COVID-19 has been slowed in many places through widespread testing of infected patients’ contacts. But the EU IVDR could mean the end of such early preventive action and much more rapid spread of such infections in future, unless action is taken now to amend the regulation.
Part 1 of this month’s Asian Medtech Associations Regulatory Networking discussions features Asean medtech market developments and Asian Harmonization Working Party news. Part 2 was a guest presentation on the current Brexit and EU IVDR outlook. This feature is hosted by Medtech Insight, the Asia Regulatory and Quality Consultancy (ARQon) and the Asia Regulatory Professionals Association (ARPA).
Vietnam is the focus of this brief update to the regular Asian Medtech Associations Regulatory Networking discussions, hosted by Medtech Insight, along with the Asia Regulatory and Quality Consultancy (ARQon) and the Asia Regulatory Professionals Association (ARPA).
ADVERTISEMENT