Scrip 100
Pfizer dominated the Scrip 100 rankings of the top pharmaceutical companies in the world based on full year 2022 pharmaceutical sales, driven by its COVID-19 success.
The past couple of years have generated important growth for the pharma industry thanks to its drugs and vaccines for COVID-19. But with the IRA, high inflation, rising interest rates and chilly public markets, biopharma faces challenges this year. Here, we summarize key messages from In Vivo’s Outlook 2023.
As the reality of annual results arrive, Scrip predicts how the top of the pharma league table is likely to change.
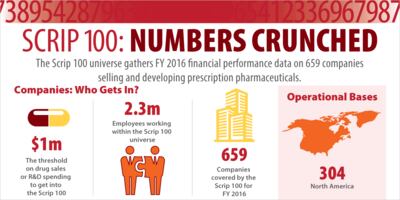
This year's Scrip 100 collection is bigger than ever, encompassing 2016 full-year financial data from more than 650 biopharmaceutical companies.
Bolt-ons or big-ticket buys? As another year passes without a big pharma mega merger despite industry giants sitting on large cash piles, we consider whether 2018 will bring major upheaval in the industry landscape, and where M&A will be most likely to take place.
Bellwethers tell you something, but to gauge the health of the pharmaceutical industry, you really need the Scrip 100. For FY 2016, it looks like drug sales are up again and profits are down. But is this what's actually going on?
Spending on R&D increased substantially in 2016; but the picture is complicated and more than half of the increase is due to accountancy updates following drug program failures, one large company's commitment to new projects, and young companies fuelled by generous financial markets.
You could be forgiven for thinking that Brexit was the only story in town on the European regulatory front in 2017, but there were of course plenty of other important developments during the year.
You could be forgiven for thinking that Brexit was the only story in town on the European regulatory front in 2017, but there were of course plenty of other important developments during the year.
Bayer's pharma president and board member Dieter Weinand talks to Scrip about the importance of big picture thinking when weighing the value of innovative drugs to healthcare systems, the revolutionary potential of big data and how "transparency and open dialogue will further enhance the understanding of our business."
Nearly 18 months after the UK voted to leave the European Union, pharmaceutical companies and regulators are still in the dark as to what kind of regulatory arrangements will exist between the two parties after Brexit takes place in March 2019.
Sanofi's chief scientific officer Gary Nabel, who joined the company in 2012 from the NIH, discusses R&D challenges for Sanofi and the wider pharma industry, and highlights the company's biggest drug development achievements and toughest moments over the last few years.
Gary Dubin, senior vice president and global medical officer in Takeda Pharmaceutical Co.’s Vaccine Business Unit, talks about the trials and tribulations of vaccine R&D and how it felt to get one of the world's first human papillomavirus injections to market.
What's next for the PD-1 market, a cancer therapy area that exploded in 2017 as recently launched products secured ever more approvals? Maria Whitman, managing principal at sales and marketing firm ZS Associates, talks about challenges facing the booming immuno-oncology market and shares her predictions for 2018.
The pharmaceutical industry's manufacturing capacity for cell and gene therapies is under pressure from an ever-increasing number of life sciences companies wanting to exploit rare expertise in the sector, but CMOs and government-backed facilities are rapidly coming on-stream, hoping to develop local clusters of companies to deliver the complete "living medicines" supply chain.
Spending on medicines as a result of litigation against health authorities is soaring in Brazil. Scrip investigates the challenges firms may face in future, including greater pricing pressure, a more NICE-like health technology appraisal system and increased scrutiny over any perceived industry wrongdoing.
Survey of recent literature shows breadth and quality of early research activity aimed at developing biomarkers to predict response to PD-1 and CTLA-4 checkpoint inhibitors.
ASCO's chief medical officer Richard Schilsky discusses innovative clinical trial designs in oncology, challenges facing developers of cancer combination therapies and future developments for the society's value frameworks.
How to make the most of the wealth of raw data in pharmaceutical R&D is an increasing challenge for the industry. The digital revolution is already having an impact in areas like clinical trials, and pharma is also starting to embrace new technologies in drug discovery.
Sub-Saharan Africa has been firmly in the regulatory spotlight over the past year or so, with east and west Africa making progress on projects to speed up drug regulatory procedures through greater cooperation and information sharing at the regional level.
ADVERTISEMENT