Ukraine
Seven “priority steps” to support Ukraine’s EU accession ambitions have been recommended to the European Commission in an open letter from Medicines for Europe, in conjunction with Ukraine’s European Business Association.
In the second part of a three-part interview with Generics Bulletin, Medicines for Europe director general Adrian van den Hoven discusses the rise in prominence of biosimilars in Europe, reflects on off-patent industry efforts to deal with crises such as the COVID-19 pandemic and the war in Ukraine, and highlights achievements such as the SPC manufacturing waiver.
Generics industry trade group Medicines for Europe has met with Ukrainian government officials to discuss how to plan for any future integration process.
Medicines for Europe has signed a memorandum of understanding with Ukraine’s European Business Association that aims to accelerate the country’s integration into the EU pharmaceutical market.
Slovenia’s Krka enjoyed a solid year for its dominant Prescription Pharmaceuticals business, with sales climbing by 7% on the back of growth for all six of its geographic business units.
More than one year into the conflict, pharma employees in Ukraine have made heroic efforts to maintain existing clinical studies – but what the country’s doctors and CROs really want is for international companies to start opening new trials again.
More than one year into the conflict, pharma employees in Ukraine have made heroic efforts to maintain existing clinical studies – but what the country’s doctors and CROs really want is for international companies to start opening new trials again.
On the anniversary of Russia’s invasion of Ukraine, the generics industry reflects on its contributions to relief efforts as well as the impact that the war has had on the supply chain.
With a suite of vastly expensive and advanced cystic fibrosis therapies entirely under its command, Vertex is facing more heat from patients and advocates in several major low- and middle-income countries.
The worst of COVID-19’s disruption appears to be in the pharma supply chain’s rearview mirror, but logistics stakeholders still have a number of questions to answer and challenges to overcome as they attempt to become future-proof.
Start-up Esper Bionics has designed a prosthetic hand that can anticipate a user’s actions, changing the game for amputees.
The Russian clinical trials organization ACTO says the “gloomy” prospects for multinational clinical trials in the country have led many sponsors to postpone the initiation of new studies or abandon them entirely in favor of other markets.
Agency tells former OOPD director Tim Coté it doesn't have the authority to consider sponsor’s country of origin when determining whether to act on applications. Despite the denial of petition, FDA continues to support Ukraine.
The agency tells former OOPD Director Tim Coté that it does not have the authority to consider the sponsor’s country of origin when determining whether to act on an application.
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have since stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
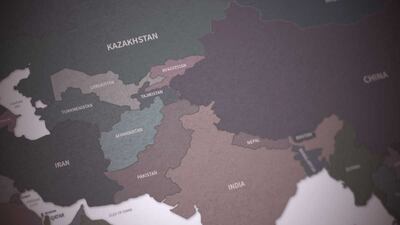
Russia and Ukraine had been key players in the pharma markets of Central Asian and surrounding countries before the launch of the Russian invasion into its neighbor on 24 February. Because of this, both countries are poised to give ground in this growing regional market.
Pharmaceutical leaders are responding to the many and varied challenges arising from the war in Ukraine.
Decentralized trials are a way to help displaced Ukrainians remain in studies and could encourage greater participation from individuals in other countries, those overseeing sites in Ukraine say.
Slovenia’s Krka surprised the market with nearly 10% group turnover growth in the first quarter, driven by increases in all six of its sales regions, “most key markets, and by all product and service groups.”
ADVERTISEMENT