Canada
Avista VC acquires OTC CMO Trillium; FDA deputy commissioner Jones to open CRN conference; AG1 founder steps down, COO moves up to CEO; ChromaDex hires general counsel; Ajinomoto, Shiru partner to develop sweet proteins; and change at ingredient provider LBB helm.
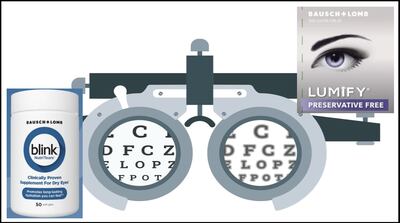
Brimonidine tartrate eye drops remain key sales driver among Canadian firm’s consumer health lines with 12% growth in the latest quarter. B+L expects pending launch of preservative-free line extension to sustain Lumify as a driver as generics of original formulation are approved.
Martha Stewart sells supplements on iHerb; Cheribundi partners spread the brand at Paris Games; GoodSport’s latest partner is a Bear; Herbalife-sponsored runner competes in ultramarathons; Dutch researcher joins Unicity science board; and Beacon Wellness adds sales strategy chief.
“Clear Eyes is a perfect example where we're dual-sourced and two facilities don't usually go down at the same time. This was just a quite an anomaly,” says CFO Christine Sacco, noting PCH works with more than 100 contract manufacturers.
NOW’s “curated collective of experts” for advice and wellness tips; Women In Nutraceuticals launches awards program; and Promino builds brrand ambassador roster.
Latest moves on selling to consumers using GLP-1 receptor agonist drugs for obesity come from firms as large as global food products manufacturer and marketer Nestle and as small as startup Promino and as ambitious as men’s health product marketer Ro offering an app at no charge to find GLP-1 drugs.
Direct seller reports Q1 sales up 1% to $1.3bn but net sales off 17.1% from year-ago period to $24.3m. Its distributor ranks jumped so far in 2024, particularly in China, where changes were made in the past year including new company leadership.
Blink NutriTears supplement containing lutein/zeaxanthin, curcumin and vitamin D3 coming in 2024 is example of a cross-segment sales benefit B+L sees in dry eye product category. Consumer eye care and vision health product sales are key drivers for 18% Q1 net sales growth to $1.1bn.
Canadian firm received approval from FDA for NDA submitted in May 2023 for Lumify Preservative Free. A month earlier, FDA approved Dr. Reddy's ANDA submitted in July 2021 for an equivalent of Lumify’s formulation, brimonidine tartrate 0.025% ophthalmic solution.
US OTC drug and supplement firms’ reports of results for the first three months of 2024 began on April 19 with P&G. JP Morgan analysts say while “some retailers in the US in particular” are reducing consumer health inventories, for the overall sector they expect “a healthier balance of positive volume and lower pricing contribution.”
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
“In recent years, this stock has performed terribly,” Wright Investors analysts note after Neptune Wellness receives notification Nasdaq would delist its common shares because share price closed below $1 for 30 consecutive trading days. Firm puts “employees, including certain key executives, on mandatory unpaid leave of absence.”
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
DeSimone returns as Herbalife CFO; Mannatech CEO Bala leaving on 1 April with Fredrick moving to post; CHPA lifetime achievement award for former Perrigo exec Needham; comedian hacks healthy nutrition for Quest; “John Wick” inspires Jacked; NBA player assists Earth Splendor; HRG owner group expands with HR director.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.
Drew Barrymore is brand ambassador for Dr. Kellyann menopause supplement; Next In Natural adds head to expanded M&A division; Mountain Valley MD appoints CFO; former Fitbit executive heads Somnee wearable firm StimScience; and PanTheryx expands scientific advisory board.