Compliance
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
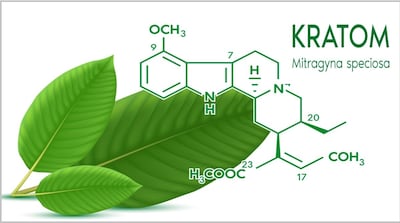
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.
Noting similar warnings a year ago, the FDA and FTC announce warnings to six more, part of a joint effort to stop sales of copycat food products containing delta-8 THC, saying companies selling these "illegal products are demonstrating complete neglect for consumer safety.”
Committee’s report published with FY2025 appropriation states a different approach to establishing FDA regulation of non-drug products containing hemp as a derivative of cannabis de-scheduled as controlled substance in the US since 2018.
CRN gained approval of motion for expedited briefing when it notified Second Circuit it would appeal District Court for Southern New York’s ruling against its motion for preliminary injunction against state law prohibiting sales to minors of supplements and OTC drugs containing ingredients labeled or promoted for weight loss and bodybuilding.
Finished product following Reagan-Udall food safety programs review establishes Human Foods Program in commissioner’s office while also realigning centers, offices and divisions across agency to improve collaboration with regulatory affairs, which conducts facility inspections and other field operations.
Where a US hemp product firm stands on whether de-scheduling the botanical as a controlled substance in the 2018 farm bill left a loophole allowing chemically derived and potentially intoxicating cannabinoid strains to qualify could determine whether it agrees with the detour.
Ag committee members in 23 May markup likely to broach topic of delta-9 THC limit for hemp. Since hemp was de-scheduled in 2018, cannabinoids other than delta-9 but with psychoactive effects have become leading sales drivers. “Most states now have legal marijuana programs, they just don't know it,” says cannabis industry attorney.
Court ruling that state’s restrictions “may very well regulate protected speech”’ allows CRN “to move forward on the merits of the case,” says CEO Steve Mister. CRN’s allegations “plausibly support the inference that the [restriction] might very well regulate protected speech,” judge writes.
Without additional funding, FDA’s ORA faces challenges in retaining and hiring staff, which will impact inspections, says office chief Michael Rogers.
Southern New York district judge rejects CRN’s arguments to block regulation from taking effect, but agrees with trade group and its members that they have standing to challenge the regulation. CRN complaint challenging law will continue in the district court.
Without FDA regulatory pathway for hemp’s lawful use in supplements and food, some states are imposing more-stringent regulations while others allow sales of intoxicating ingredients not considered controlled substances as hemp derivatives.
US Cannabis Council says farm bill reauthorization is “key opportunity to tackle the national crisis caused by unregulated intoxicating hemp products” by limiting the variety of hemp derivatives which qualify as de-scheduled.
Dietary supplements containing unsafe or unauthorized substances were reported to the European Commission by national regulators on around 60 occasions in the first quarter of 2024.
FDA ODSP explains that whether information will be deemed a trade secret or otherwise confidential commercial information inaccessible to other firms in an NDIN master file is subject to Freedom of Information Act regulations and the relevant Food, Drug and Cosmetic Act rule.
Some trade groups couldn’t agree more that spurring NDIN compliance will benefit the supplement industry, but few in industry are convinced the agency will strengthen enforcement of the requirement or across supplement sector generally.