Compliance
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
Children with ADHD often struggle with bedtime due to restless minds, difficulty winding down, and heightened sensitivity to nighttime distractions. This article explores the reasons behind these sleep challenges and offers practical solutions.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Analysts suggest more and larger investments than president’s recent $1m and $5m private placements needed to last long enough to establish sales of its functional beverage sufficient to turn a profit. Results expected soon from clinical trials on “detoxifying the alcohol out of” users’ bodies.
Without additional funding, FDA’s ORA faces challenges in retaining and hiring staff, which will impact inspections, says office chief Michael Rogers.
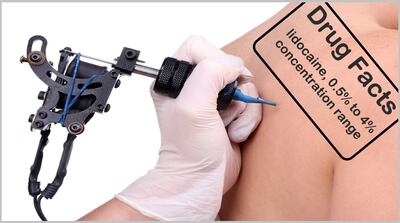
FDA warns six businesses found selling directly to consumers topical formulations containing lidocaine outside the 0.5% to 4% concentration range allowed under OTC monographs for topical analgesics
Agency wants more information about API suppliers as it winds up case against KV Tech for hidden use of Dr. Reddy’s plant and seeks to find disappearing manufacturer of contaminated OTC eye drops.
FDA says FivFivGo, Rebright and South Moon are copycats in packaging easily mistaken for Lumify OTC eye drops. Testing found South Moon contaminated with bacteria linked to an antibiotic-resistant infection; agency warns using any of the three poses risk of eye infection.
Oversight and Investigations Subcommittee conducts hearing as subcommittee chair Morgan Griffith and Energy and Commerce chair Cathy McMorris Rodgers leave no doubt they give FDA failing grade on foreign inspections. Five foreign facilities among firms in FDA’s latest warnings.
PDC Brands declines to provide support for nine challenged claims for Dr. Teal’s line of balms, lotions, oils, soaps, sprays and other topicals. It continues using those after halting use of 25 other similar claims challenged by P&G, marketer of melatonin-containing ZzzQuil.
All lots of nearly 30 different dry eye and lubricating products made by Mumbai-based Kilitch Healthcare India and sold in US by major drug store chains and marketed under multiple private label brands are recalled after FDA investigators found insanitary conditions at firm’s facility.
Smart Women’s Choice agrees to consent decree requiring it to halt production and sales of namesake brand product promoted as being hormone-free and with claims including “immobilizing sperm” and “preventing fertilization.” Related company with similar name, product and claims continues sales.
FDA also responds to sting OTC eye drop sector felt with warnings to Amazon after investigators purchased drops labeled with violative claims and a natural health care products firm with Amazon storefront offering drops with ingredient commonly used in joint health supplements and claims to “soften the membranes of the eye.”
Since FDA provided draft guidance on quality considerations for ophthalmic drugs, steady stream has continued of OTC brand recalls due to manufacturers’ failure to ensure sterility in production processes and purity of ingredients and other components.
Need for guidance shown not only in recalls over past year of OTC eye drops due to bacterial contaminants found in products and insufficient sterility practices in facilities but also in warning letters sent in 2022 and 2023 to firms manufacturing Rx and OTC ophthalmic drugs.
Recall starts after products across OTC indications, including at-home pregnancy and marijuana tests, “were stored outside of labeled temperature requirements” and “inadvertently shipped to certain stores” in 23 states.
One of firms targeted in eight most recent warnings published since early September to OTC drug manufacturers, Zhao Qing Longda Biotechnology Co. in China, was dinged for both failing to respond to a request for records and for concerns about its production with DEG and EG.
“Choosing which claims to make,” an advertiser “affects the amount of substantiation required. A structure function claim, supports digestive health, may require a different level of substantiation than if you choose to make the claim prevents diarrhea,” says FTC attorney Christine DeLorme.
FDA drug user fee program chief tells OTC industry stakeholders accurate information in electronic Drug Registration and Listing System is key to setting annual facility registration user fee rates. CDER publishes warning letters to US retailer and a South Korean manufacturer noting noncompliance with eDRLS requirements.