United States
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
Global beauty giant is working to adapt its approach to the prestige market to meet escalating competition, increased points of sale, and more knowledgeable consumers with new shopping habits, CFO Tracey Travis said at the Barclays Global Consumer Staples Conference.
Court order dismisses claims against Perrigo and Joseph Papa, its former CEO, with prejudice. Settlement also bars any claim against plaintiffs and class members, “whether arising under state, federal, common or foreign law,” alleging liability for losses.
Proposal to have panelists rate the strength of efficacy and safety evidence on a numeric scale also draws support in written comments on ways to optimize the advisory committee process.
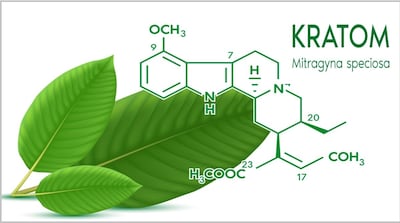
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
CEO Sue Nabi says Coty has created a “multi-functional, stand-alone organization” within its Consumer Beauty business to drive rapid-fire innovation. In a 20 August presentation, Nabi said the firm’s innovation pipeline undergirds its fiscal 2025 outlook of 6%-8% growth.
Conflicted experts should be allowed to participate as nonvoting members and panels should take a benefit-risk vote on product-specific applications, the majority of respondents said in a survey conducted by 3D Communications.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
Height of plaintiff attorney’s argument to present evidence which would prompt speculation by a jury was request to parse research by Kenvue’s lead expert, who coordinated the International Consensus Statement on ADHD by the World Federation of ADHD, where he’s president.
Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”
In this installment of HBW Insight’s “Inside Regulatory Affairs” series, we hear from Sharee Crumbey and Monica Sharda, regulatory affairs specialists at The Honest Company, about the mounting challenges and rewards of the job.
A change in leadership of the US FDA device center is taking place with an eye towards stability in the longer term, but the upcoming elections could lead to more disruptive personnel changes.
David Ball moves from Bayer’s North America business to be Perrigo’s first chief brand and digital officer. He joins CEO who also moved to Perrigo from Bayer with decades of branded product experience.
The Fragrance Creators Association president and CEO discusses the group’s vision for fragrance industry stewardship and opportunities provided by AI, biotechnology and wellness trends in an exclusive Q&A with HBW Insight.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to CHPA’s Mike Bailey, senior vice president of regulatory and scientific affairs; and regulatory and scientific affairs VPs Marcia Howard and Jay Sirois.
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
The FTC says its final rule banning fake consumer reviews and testimonials will help to restore its depleted toolbox for obtaining civil penalties. The agency maintains the final rule’s benefits will greatly outweigh costs for honest companies increasingly set back by bad actors in the space.