Manufacturing
The gut’s microbiome will shape future medical advances, offering personalized therapies and prevention strategies for a wide range of diseases.
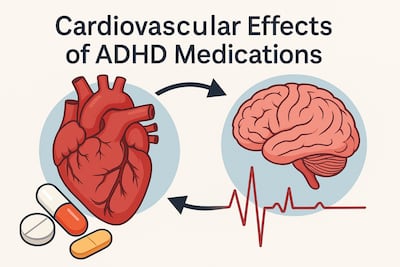
A look at new research on the cardiovascular effects of ADHD medications and what it means for clinical care. This article explores the latest clinical and epidemiological research on the cardiovascular effects of medications commonly prescribed for ADHD, examining potential heart-related risks, underlying mechanisms, and their implications for patient safety, clinical decision-making, and long-term treatment strategies across diverse age groups and risk profiles.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”
“We continue to believe that taking care of your health or someone in your family is the last place to look to make a change or save a few pennies,” says CEO Ron Lombardi. Firm more frequently using air freight services so Clear Eyes products are on store shelves when consumers expect.
Church & Dwight’s slower sales at retail in June-July than in January-May point to still slower results for rest of year. It doesn’t expect second-half help from gummy vitamin lines or give them a full vote of confidence for remaining in portfolio.
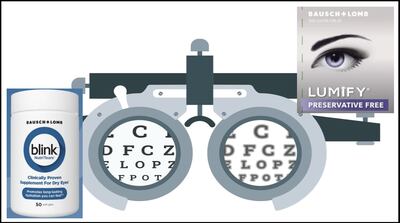
Brimonidine tartrate eye drops remain key sales driver among Canadian firm’s consumer health lines with 12% growth in the latest quarter. B+L expects pending launch of preservative-free line extension to sustain Lumify as a driver as generics of original formulation are approved.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.
FDA reminds companies which registered with agency solely to manufacture OTC sanitizers during COVID-19 public health emergency they will be subject to FY2025 OTC monograph user fees if they don’t delist and deregister as monograph drug manufacturers by 12 a.m. on 31 December.
House and Senate reports again reference sunscreen but add admonishment that FDA “harmonize its approach with international testing standards.” Item exclusive to House report encourages guidance for drug firms on successfully submitting an application for Rx-to-OTC switch of an oral contraceptive.
Committee’s report published with FY2025 appropriation states a different approach to establishing FDA regulation of non-drug products containing hemp as a derivative of cannabis de-scheduled as controlled substance in the US since 2018.
USDA food and nutrition lead Dean joins Global Food Institute as executive director; supplement, skin care entrepreneur Ishaq leads Amare; Solarea Bio appoints pharma sales veteran CCO to push medical foods to market; two-time HFSPO grant winner named president; and Natural Grocers CFO retiring.
The European Chemicals Agency’s committees for Risk Assessment (RAC) and Socio-Economic Analysis (SEAC) are evaluating sector by sector the proposed REACH restriction on PFAS and more than 5,600 public comments, reaching provisional conclusions on cosmetics at their June meetings. A date has not been set to consider PFAS uses in medical devices, which are numerous, essential, and in many cases devoid of alternatives, industry says.
The US FDA has issued its final guidance defining manufacturer behaviors it deems as hampering the agency’s ability to conduct an inspection. In a previous draft guidance, the agency expanded its longstanding policy on inspections of drug companies to include device makers as well.
Attorneys for some plaintiffs alleging harm from talc products J&J marketed ask a court to order the firm, along with a subsidiary holding company it created in 2021 to manage its talc liabilities and immediately placed in bankruptcy, to provide emails and other communications among in-house counsel and compel their testimony.
More than a decade after acquiring vitafusion and L’il Critter gummy vitamin brands and touting gummy as a key growth driver in the VMS space, C&D’s seeing different market trends.
FDA asks for nominations for voting or non-voting consumer members of its advisory committees. Also wants to hear from consumer organizations interested in participating in selecting consumer representatives for its advisory committees or panels.